Managing Soil pH for Crop Production in
Calcareous-Alkaline Soil

Introduction
In semiarid soils of the Western U.S., altering soil pH is not easily accomplished nor straightforward. Recall that pH is a measure of the acidity or alkalinity of a substance. More specifically, Utah’s soil pH range can be 1,000 times more acidic or alkaline than neutral (7.0) pH soils. For example, a pH change by one unit equals a tenfold change in acidity or alkalinity (a soil with a pH of 8.0 is 10 times more alkaline than soil with a pH of 7.0).
In semiarid regions, typical high-pH soils are also calcareous, meaning there is a large amount of solid calcium carbonate (lime) in the soil. When soil-acidifying amendments are added to these soils, the lime dissolves and counteracts any of the applied soil-acidifying amendments. This fact sheet explains how to identify whether crop symptoms are related to soil pH, how to perform an in-field test for soil pH buffering capacity, and what can or cannot be done to alter soil pH in calcareous soils.
Quick Facts
- The pH scale ranges from 1.0–14.0.
- pH values below 7.0 are acidic and values above 7.0 are alkaline.
- Most soils have a pH between 4.0 and 10.0.
- A pH change by one unit equals a tenfold change in acidity or alkalinity. For example, a pH of 8.0 is 10 times more alkaline than soil with a pH of 7.0.
- Semiarid soils are usually alkaline.
- Attempts to lower calcareous-alkaline soil pH are often futile or impractical.
Importance of Soil pH
Soil pH is one of several soil chemical properties that regulate plant-nutrient availability in the soil (Hartemink & Barrow, 2023; Truog, 1947). Common crops like alfalfa, corn, and small grains tolerate acidic and alkaline soils between pH’s of 5.7 and 8.1. Beyond this range, these crops develop various nutrient deficiencies. Other plants, like blueberries, are more limited by pH and will not grow well in soils with a pH > 6.0. Variation in tolerance occurs due to plant-available nitrogen, phosphorus, and micronutrients such as iron, boron, manganese, copper, and zinc, which can be severely reduced in moderate to high pH conditions, even though these nutrients are present in most soils (Truog, 1947). This is the main reason iron deficiency (iron chlorosis) is a widespread problem for specialty and ornamental crops planted in calcareous soil (Figure 1) (Bloom & Inskeep, 2007).

Figure 1. Comparing Healthy (left) and Iron-Deficient (right) Autumn Blaze Maple (Acer x freemanii 'Jeffersred') Leaves from Ogden, Utah
Note. Iron chlorosis is a common ailment for this hybrid maple in Utah.
Effect of Calcium Carbonate (Lime) on Soil pH
Decreasing the pH of calcareous soil is a challenge because the soil is so well buffered. In Utah, 15% to 40% of soil is calcium-lime by weight. On a per-acre basis, each percent of lime weighs approximately 10 tons in the top 6 inches of soil, or one acre-furrow slice. Consequently, a typical acre-furrow slice in Utah contains a 150- to 400-ton reservoir of lime. Until completely consumed, the lime will buffer and neutralize the strongest of acids (De Vries et al., 1989). For example, it takes 20,000 to 50,000 gallons of pure sulfuric acid to dissolve an acre furrow slice of 15% to 40% lime before a pH change can occur (Cardon, 2018).
Calcareous Soil Acidification Research in Utah
Experiments from Utah between 1995 and 1999 all suggested that pH acidifying amendments applied to calcareous soil do not effectively lower soil pH long-term. Short summaries of these studies are included below. The science has not changed concerning soil acidifying amendment applications in high pH soils; therefore, no further studies have been performed.
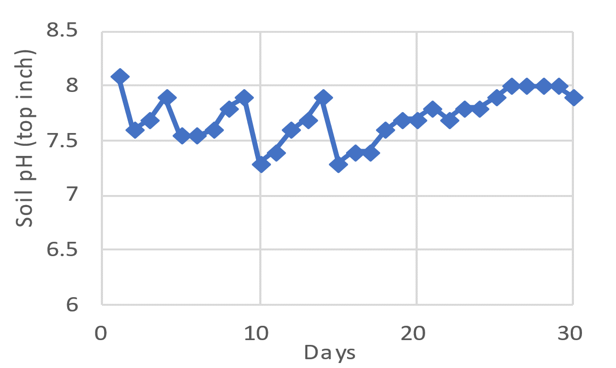
Figure 2. Repeated Liquid Sulfuric Acid Application Effects on Soil’s Surface-Inch pH
Notes. Acid was applied four times (days 1, 4, 9, and 14) at the rate of 1,000 pounds of sulfur per acre per application. Soil pH was measured daily.
Liquid Sulfuric Acid Applications
In 1999, a field experiment in Cache County, Utah, demonstrated lime’s buffering capability. One thousand pounds per acre of sulfuric acid (98%) was repeatedly sprayed onto soil containing 38% lime with an initial pH of 8.1. Soil pH was measured daily in the surface inch of soil (Figure 2). From each application of sulfuric acid (days 1, 4, 9, and 5), soil pH decreased to about 7.5 and rebounded to 8.0 within 2 to 10 days due to the remaining lime (Figure 2) (Gale, et al., 2001).
Elemental Sulfur Applications at Four Rates
Elemental sulfur (90% to 99% sulfur) oxidizes slowly to form sulfuric acid in soil. A 1996–1999 field experiment in Weber and Cache counties evaluated the effect of solid elemental sulfur additions from 0 to 10,000 pounds per acre on soil pH. Results demonstrated that the Weber soil with no lime could be acidified while pH was only slightly decreased on the high lime soil in Cache (Figure 3). At both locations, measurable changes in soil pH were not observed until 12 to 18 months after sulfur application. It is important to note that at the Weber location, the 5,000 and 10,000 pounds of sulfur per acre killed the alfalfa because the soil became so acidic. Furthermore, at the Weber site where soil pH was lowered, the cost of the 1,000-pound rate of elemental sulfur at $450 per ton (average of 2021 and 2022 prices) would have been about $225 per acre to lower pH from 7.0 to about 6.0.
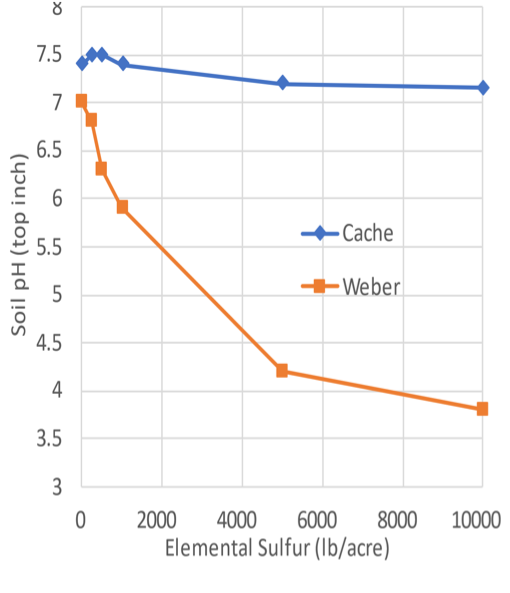
Figure 3. Elemental Sulfur Effects on Soil pH in the Surface 6 Inches
Notes. The Weber County (Huntsville) soil contained no lime, while the Cache County (Logan) soil contained 38% lime. Both fields were in alfalfa, and the alfalfa died at the Weber location, with 5,000- and 10,000-pound sulfur/acre treatments.
Sulfur Burner Irrigation Water pH Treatments
In 1995, a level basin irrigation study conducted in Millard County, Utah, diverted approximately 5% of a canal’s water stream through a sulfur burner. The treated water was then reintroduced into the canal where it mixed with the mainstream before entering the field. During four irrigations the sulfur burner acidified the water and removed all lime. Tables 1 and 2 show the results when treated and untreated waters were used to irrigate adjacent basins containing alfalfa. The sulfur burner treatment did not increase alfalfa yield, alfalfa crude protein, or alter soil pH and available nutrient levels.
Identifying Soil pH Problems
Overapplication of soil amendments can often cause more harm than good. Generally, plants exhibit similar symptoms of stress in both alkali and alkaline soils. Before investing in soil acidifying amendments to lower soil pH, consider whether pH is a problem (Figure 4).
It is important to note that alkali and alkaline soils are not the same soil type. Over time, soils become alkali by accumulating excess soluble mineral salts, i.e., calcium, magnesium, potassium, and sodium cations. These soils sometimes have a pH greater than 7.0 and are also alkaline.
The bottom line is that alkalinity is measured by pH and salinity (or alkali) is measured by electrical conductivity (EC). See Managing Saline and Sodic Soils and Irrigation Water (Barker et al., 2023) for more information about measuring and managing soil salinity.
Table 1.
Irrigation Water Quality Parameters Before, During, and After Sulfur Burner Irrigation Water pH Treatments
|
Time |
pH |
Sodium |
Calcium+ |
Bicarbonate |
Adjusted SAR |
|
---------Milliequivalents per liter---------- |
|||||
|
Before |
8.0 (0.1) † |
10.4 (0.5) |
8.7 (0.4) |
5.0 (0.3) |
12.0 (0.6) |
|
During |
2.3 (0.2) |
10.1 (0.7) |
9.1 (0.7) |
0.0 (–) |
- |
|
After |
7.4 (0.2) |
10.5 (0.5) |
9.1 (0.5) |
4.6 (0.1) |
11.5 (0.5) |
† Mean (standard error) of four samples collected from irrigations in April, May, June, and August
Table 2.
Comparing Untreated and Sulfur-Burner-Treated Water on Alfalfa Yield, Crude Protein, and Soil Chemical Properties at Growing Season’s End
|
Alfalfa and soil properties |
Untreated control |
Sulfur burner treated |
|
Yield (tons/acre) |
6.6 |
6.5 |
|
Average crude protein (%) |
20.2 |
20.9 |
|
Soil pH |
8.0 |
7.9 |
|
Soil test phosphorus (ppm) |
4.2 |
5.3 |
|
Soil test zinc (ppm) |
0.7 |
0.7 |
|
Soil test Iron (ppm) |
6.8 |
6.8 |
|
Soil test copper (ppm) |
1.1 |
1.1 |
|
Soil test manganese |
2.3 |
2.4 |
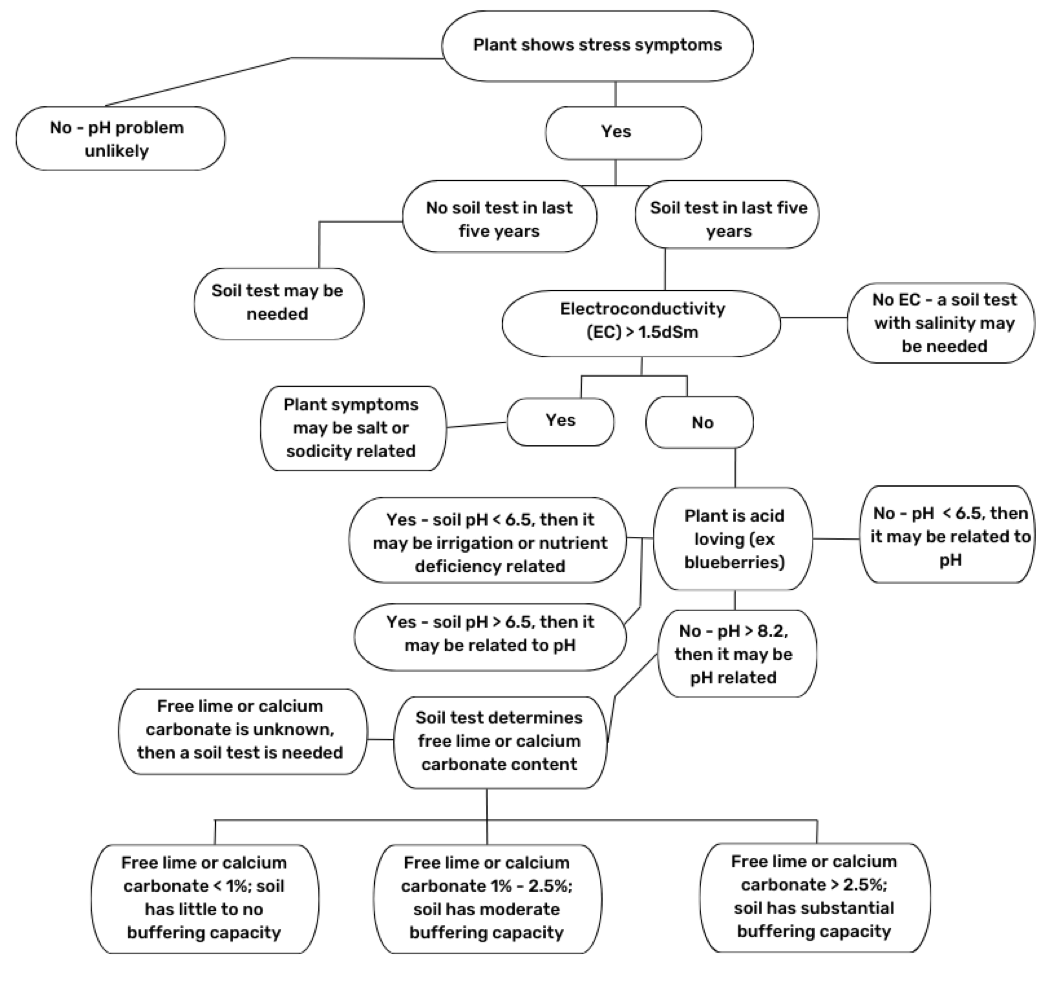
Figure 4. Conditions That May or May Not Warrant Soil pH Adjustment to Improve Crop Health
In-Field Identification of Calcareous Soil

Figure 5. Soil and Vinegar Reactions on Soil
Note. Bubbles form as CO2 gas is released during the reaction: rapid bubble formation (top); slower bubble formation (middle); and no bubbles or fizz (bottom).
Relative soil lime can be quickly determined in the field. This test requires vinegar and a spoon or eye dropper.
Step 1. Use a field soil sample to form a pea to golf-ball-sized soil ball (it’s okay if it breaks apart). Place the ball where vinegar drops won’t make a mess.
Step 2. Use the spoon or eye dropper to add a little vinegar to the soil ball (a few drops will do for smaller balls; large balls may need more vinegar to react).
Step 3. Observe the reaction for 30 seconds.
- High lime soils will fizz or bubble (like carbonated beverages) for several seconds. This reaction means high buffering capacity, and lowering pH is not feasible.
- Moderate lime soils show weaker fizzing or slower bubble formation than high lime soil. This reaction means moderate buffering capacity, and lowering pH may be economically impractical and unlikely to provide permanent results.
- Low to no lime soils present no reaction. It’s recommended that these soils receive further testing to determine free lime or calcium carbonate content before any attempts to lower soil pH; contact the local Utah State University (USU) Extension office for more information.
Managing Soil pH Problems
Given the impracticality of lowering pH in calcareous soil, more prudent options include leaching excess salts
or adding prescribed amounts of micronutrients such as zinc or iron to manage nutrient deficiencies. Table 3 provides general pH guidelines to assist the decision process for soil remediation.
Farming the Soil. Cultivation, cropping, irrigation, and returning crop residues (organic matter) to the
soil may, over time, slightly lower pH. Native (uncultivated) desert soils commonly have pH values in the
range of 8.0 to 9.0. It is common to see soil pH decline 0.2 to 0.5 units after several years of farming. Increasing organic matter returned to the soil can accelerate this process. However, to prevent additional problems, e.g., excess salt or nutrient accumulations, apply organic matter according to laboratory test recommendations.
Applying Elemental Sulfur and Liquid Acid. Band elemental sulfur or liquid acids to lower pH in small volumes of soil for row crops or where lime concentration is relatively low (< 2.5%) instead of attempting to change pH in the entire acre furrow slice.
Adding Gypsum (Calcium Sulfate) to the Soil. Gypsum has no direct effect on soil pH. Gypsum’s main use is in reclaiming sodic alkali soils.
Providing Irrigation Water Treatments. Many irrigation sources in the Intermountain West have high pH coupled with high levels of carbonate, bicarbonate, and sodium. Sulfur burners and direct injection of acids into irrigation water has the potential to improve irrigation water quality, aid in reclaiming sodic soils, and improve soil water infiltration and drainage characteristics. However, these treatments cannot meaningfully lower soil pH. Greenhouses, golf courses, and orchards have successfully used direct acid injection and sulfur burners to treat irrigation water. This is apparently due to the smaller volume of water used by these operations and the ability to treat a larger proportion of the water.
Table 3.
General Guidelines for Interpreting Soil pH in Utah
|
Soil pH |
Interpretation |
Recommendation |
|
Less than 6.0 |
Acidic. Rare property in Utah. May indicate the presence of reduced iron or sulfur compounds or contaminants in soil. For information on soil contamination, see Trace Element Contamination in Urban Soils: Testing and Management (Stock et al., 2022). |
Monitor pH every 3–5 years, or more frequently if problems develop. If pH continues to decline below 6.0, lime additions may be needed. |
|
6.0–7.5 |
Near neutral. Ideal pH for most crops. |
Monitor pH every 3–5 years as part of a regular soil testing program. |
|
7.6–8.2 |
Moderately alkaline. Typical soil pH range for Utah. Should not pose significant problems for crop growth. |
Monitor soil pH every 3–5 years as part of a regular soil testing program, or more frequently if problems develop. |
|
Above 8.2 |
Highly alkaline. May cause plant growth and water infiltration challenges. Soil may be sodic (contain excess sodium). |
Test soil for sodium absorption ratio (SAR) to determine sodicity. Options are also available, see Managing Saline and Sodic Soils and Irrigation Water (Barker et al., 2023). |
Summary
There are a few things to consider before attempting to alter soil pH. Does pH management meaningfully benefit agronomic outcomes, project scale, project feasibility, and economic cost to benefit ratio? Furthermore, do other management options, such as leaching excess salt or nutrient management, achieve similar outcomes? Finally, care should be taken to address safety, environmental quality, and crop health concerns before applying soil pH-adjusting amendments.
References
-
Barker, B., Cardon, G., Yost, M., Stock, M., Creech, E., & Gale, J. (2023). Managing saline and sodic soils and irrigation water [Fact sheet] Utah State University Extension. https://digitalcommons.usu.edu/extension_curall/2357/
-
Bloom, P. R., & Inskeep, W. P. (1986). Factors affecting bicarbonate chemistry and iron chlorosis in soils, Journal of Plant Nutrition, 9(3-7), 215–228. DOI: 10.1080/01904168609363438
-
Cardon, G. (2018). Why are my soils so alkaline? Can I lower my soil’s pH? Dirt Diggers Digest, 2(3). https://extension.usu.edu/dirtdiggersdigest/soils-alkaline
-
De Vries, W., Posch, M. & Kämäri, J. (1989). Simulation of the long-term soil response to acid deposition in various buffer ranges. Water, Air, and Soil Pollution, 48, 349–390. https://doi.org/10.1007/BF00283336
-
Gale, J., Koenig, R., & Barnhill, J. (2001). Managing Soil pH in Utah [Fact sheet]. Utah State University Extension https://digitalcommons.usu.edu/extension_curall/923
-
Hartemink, A. E., Barrow, N. J. (2023). Soil pH - nutrient relationships: the diagram. Plant and Soil, 486, 209–215. https://doi.org/10.1007/s11104-022-05861-z
-
Rogovska, N. P., Blackmer, A. M., & Mallarino, A. P. (2007). Relationships between soybean yield, soil pH, and soil carbonate concentration. Soil Science Society of America Journal, 71(4), 1251–1256
-
Turog, E. (1947). Soil reaction influence on availability of plant nutrients. Soil Science Society of America, 11(C): 305–308.
Photos and figures were provided by the authors.
Published December 2023
Utah State University Extension
Peer-reviewed fact sheet
Authors
Jody Gale, Rich Koenig, and James Barnhill (2001)
Revised 2023 by Cody Zesiger, Jody Gale, Matt Yost, and Grant Cardon
Related Research























































