Fertigation Facts

Introduction
Fertigation is the application of fertilizer through an irrigation system (Fig 1). It can be implemented in surface, sprinkler, and drip systems. In the 2013 agriculture census, nearly 135,000 acres of irrigated cropland in Utah utilized fertigation (USDA-NASS, 2014). Utah growers most commonly fertigate corn (33-41% of the total irrigated corn acres) and orchards (37% of total irrigated acres), but it is also used to a lesser degree on small grains, alfalfa, and other hay (9-23% of the total irrigated of these crops).
In most cases, fertilizer used for fertigation is available in liquid solutions or in a soluble form. Liquid fertilizer such as Urea Ammonium Nitrate (UAN), Ammonium Thiosulfate (ATS), Ammonium Polyphosphate (APP), and Anhydrous Ammonia (NH3) are most commonly used due to their convenience, and are currently the primary forms sold by fertilizer companies for fertigation in Utah. In addition to the liquid fertilizers, soluble fertilizers are an additional option for supple- menting crops during the growing season. A variety of soluble products are available at local agronomic retailers.
The purpose of this fact sheet is to provide general information on forms of fertigation for primary plant nutrient, fertigation timing, and fertigation economics.
Nitrogen Fertigation
The most common nutrient that is applied by fertigation in Utah is nitrogen (N). Nitrogen fertilizers have a high solubility, which makes them relatively easy and effective to apply with an irrigation system. Because of its high solubility, N is also extremely susceptible to leaching.
There are several different forms of N that can be used for fertigation. One of the most commonly used in Utah is UAN (32-0-0). The nitrogen in UAN is in three forms - 50% urea, 25% ammonium, and 25% nitrate (Fernandez, 2016). Anhydrous Ammonia (NH3, 82% N) is commonly used in surface irrigation systems because it can be bubbled into the irrigation water (Fig 2). Anhydrous Ammonia is less expensive than soluble liquid nitrogen per unit of N, and is a common option for surface irrigators. Be aware that anhydrous ammonia typically increases the pH of the water around the application site, and that N losses from volatilization can be as high as about 30-50% of the N applied, which can also cause poor application uniformity (Pettygrove et al., 2009).
Great caution must be taken when using NH3 because of its high reactivity with water on the skin and organs.

Because there are many different forms of N fertilizers it is important to pick the correct one for your application. Keep in mind that not all forms of nitrogen will be immediately available to the plant. Nitrate and ammonium are the predominate forms used by plants and are usually rapidly available after application. Urea is not readily accessible and must be converted into ammonium and nitrate by soil bacteria before uptake can occur (Beegle, 2005). Conversion may take several days depending on soil conditions and temperature. These conditions should be considered when deciding fertigation timing.
When choosing a N fertilizer, look for forms that are highly soluble, less corrosive, and will meet the nutrient needs of your crop at the correct time.

Phosphorus Fertigation
The most common form of phosphorus (P) that is fertigated in Utah is APP (11-37-0). Most irrigation water in Utah is hard water containing high amounts of calcium (Ca) and magnesium (Mg). When liquids containing APP are injected into high pH water, Ca- P precipitates may form. The resulting precipitates may plug irrigation lines and emitters, decrease the life of nozzles, and increase maintenance costs. Applying phosphoric acid instead of APP is a less practiced method because it may increase wear and tear on irrigation systems; but it can usually resolve the issue of precipitates forming in the irrigation system. Due to the challenges Utah’s hard water presents, and the fact that phosphorus is not easily leached from the soil, broadcasting and incorporating (where possible) solid forms of phosphate fertilizers before the growing season is a more common practice.
Potassium Fertigation
When potassium (K) is needed according to soil tests, fertigation is an option. Liquid potassium (K) fertilizer is rare. However, most K fertilizers are soluble in water and can be used for fertigation in the right applications. The two most common used for fertigation are potassium chloride (KCl) and the more expensive potassium nitrate (KNO3) (Boman and Obreza, 2015). Potassium can precipitate when combined with other fertilizers so be sure to test small mixtures in a jar or container prior to fertigation. Although feasible, K will rarely be economic to fertigate as a stand-alone fertilizer.
Fertigation of K may be suitable in instances su ch as intensely hayed cropping systems, sandy soils (less than 10% clay content), high-value crops, and depleted soils.
With that being said, potash is one of the most inexpensive fertilizers and is easily broadcasted at the beginning of the growing season.
Water Chemistry and Fertigation Compatibility
Water chemistry can have an adverse effect on the ability to deliver liquid fertilizers through an irrigation system. For example, aqueous ammonia injected as an N source can increase the pH of the water to an extent that dissolved salts in the water may precipitate, forming solid crystals that can clog nozzles and drip emitters. High bicarbonate in waters, most commonly found in shallow groundwater well sources, can cause rapid precipitation of calcium and magnesium in fertilizer sources such as calcium nitrate or CAN 17 (a common liquid N source). Phosphate fertilizers (including ammonium poly phosphates used as N sources) are particularly sensitive to precipitation when irrigation water is high in dissolved calcium and magnesium, especially at a water pH of 7.5 or higher. Sulfate forms of various nutrients can also form gypsum or Epsom salt precipitates in high pH, high dissolved calcium and magnesium content waters. Potassium fertilizers (except sulfate of potash as just noted) rarely have issues with precipitation when injected in irrigation waters.
A simple jar test can be performed prior to fertigation injection to test for irrigation water incompatibility with liquid fertilizers. This is done by filling a glass jar with irrigation water directly from the source and at the temperature it is normally delivered to the irrigation system, and then mixing in liquid fertilizer at the desired concentration. Vigorously shake and aerate the solution for one minute and then let stand for 15 minutes. If any cloudiness in the solution forms, or one notes any solid precipitates settling to the bottom, suspended, or floating, there is significant likelihood of nozzle or emitter plugging with the chosen combination of water and fertilizer.
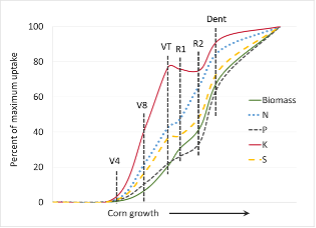
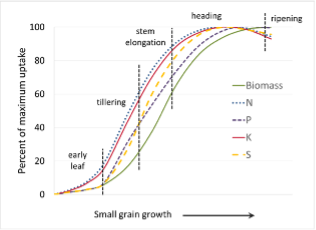
Timing of Fertilizer Application and Nutrient Uptake
Crops use different amounts of nutrients at different growth stages. For example, less than half the total N and P uptake occurs prior to the reproductive corn stages, whereas nearly 80% of the K uptake occurs prior to reproductive stages (Fig 3). Uptake of N, P, and K is more consistent for small grains and the majority of the uptake occurs during tillering and stem elongation (Fig 4). Information of this sort will help to determine optimal fertigation timing. Soil and tissue testing can help specify the crops nutrient requirements. Matching fertigation to major crop uptake periods will help maximize nutrient efficiency and increase crop yields and quality.
In high nitrogen loss scenarios, more frequent applications at lower rates of leachable nutrients may enhance your nutrient use efficiency, and save fertilizer costs. For example, Nebraska and other states recommend applying about 20-30 lb N/acre per irrigation for corn, starting with the first irrigation and ending when nitrogen uptake ceases (Ferguson, 2009).
Economics of Fertigation vs. Broadcast Applications
Few economic comparisons of fertigation vs. broadcast application of fertilizers have been conducted because of the difficulty in comparing total fertigation prices among agronomic companies. In addition to fluctuating prices of product, each agronomic company will charge differently for the various components of fertigation and the fertilizers. Agronomic companies often attempt to outbid one another and develop different pricing structures when it comes to fertilizer and fertigation services and sales. A simple method to evaluate the two methods is to use a partial budget approach. A partial budget simply evaluates the change due to the use of either fertigation or broadcast spreading. In the case below, broadcast application would be the base case and we will compare that to utilizing fertigation application. The table below provides a framework:
Table 1
Partial Budget Framework for Fertigation Application.
| Key Variable Changes | Fertigation Application | Impact |
|---|---|---|
| Yield Change | Does fertigation provide a change in yield to offset increase price? |
+ or - |
| Cost Change | Evaluate the cost per acre for each application method. This includes the cost of fertilizer, appli- cation cost, equipment cost, and maintenance costs. | + or - |
| Overall Change | Add up the changes to provide an overall analysis |
+ or - |
Additional Information
It is important to note that fertigation applications are only as uniform as the irrigation applications. Windy conditions can significantly decrease the uniformity of fertigation applications from overhead sprinklers. Thus, it is not recommended to use pivots for fertigation in windy conditions if it can be avoided.
Fertigation in furrow/flood irrigation systems is generally riskier and not as efficient as in pressurized systems. This is due to the risk of fertilizer loss in run-off water and because application uniformity can be low. Loss of fertilizer in runoff water not only represents a direct economic loss to the farmer, but also poses the risk of environmental pollution.
Effective fertigation requires careful monitoring of fertigation timing, crop growth stages, irrigation system operation, rates, and additional equipment maintenance. Keep in mind that using fertigation to apply fertilizers will require more setup procedures than simply hooking up the fertilizer cart and broadcasting across the field, and potentially more monitoring and maintenance of equipment.
However, once a fertigation system is setup, subsequent fertigations throughout the growing season should require much less effort.
For more information on setup and equipment involved in fertigation see the companion USU Extension publication titled “Chemigation Guide”.
Summary
The use of fertigation for field and horticultural crops is increasing in Utah. Fertigation can be an effective method for improving nutrient stewardship and improving crop yield and quality. Keys to successful fertigation include irrigation system maintenance and uniformity, proper fertigation setup and management, and timing nutrient applications to match crop needs.
References
Boman, B., and T. Obreza. 2015. Fertigation nutrient sources and application considerations for citrus. University of Florida Extension. Circular 1410. Available at https://edis.ifas.ufl.edu/pdffiles/CH/CH18500.pdf
California Department of Food and Agriculture. Nitrogen fertilizers and management. Available at https://www.cdfa.ca.gov/is/ffldrs/frep/pdfs/Secti on3NMP.pdf (verified 31 July 2019).
Ferguson, R. 2009. Using fertigation for efficient nitrogen application. University of Nebraska. Available at https://cropwatch.unl.edu/using- fertigation-efficient-nitrogen-application (verified 22 March 2019).
Fernandez, F.G. 2016. The three biggies: Urea, anhydrous ammonia, and UAN. University of Minnesota Extension. Available at https://blog- crop-news.extension.umn.edu/2016/05/the- three-biggies-urea-anhydrous.html (verified 22
March 2019).
Heard, J. 2006. Nutrient accumulation and partitioning by grain corn in Manitoba. In: Great Plains Soil Fertility Conference Proceedings. A.Schlegel (ed). Vol. 11. March 7-8, 2006. Denver, Colorado. p. 180-185.
Kenkel, P., & Fitzwater, B. 2015. Causes of fertilizer price volatility. Availible at: https://articles.extension.org/pages/72692/cause s-of-fertilizer-price-volatility (verified 31 July 2019).
Malhi, S.S., A.M. Johnston, J.J. Schoenau, Z.H. Wang, and C.L. Vera. 2006. Seasonal biomass accumulation and nutrient uptake of wheat, barley and oat on a Black Chernozem soil in Saskatchewan. Canadian Journal of Plant Science. 86: 1005–1014. DOI: 10.4141/P05-116
Pettygrove, S., L. Schwankl, C. Frate, and K. Brittan. 2009. Improving water-run nitrogen fertilizer practices in furrow- and border check- irrigated field crops. Final Report to California Dep. Food Ag. Available at: https://www.cdfa.ca.gov/is/ffldrs/frep/pdfs/com pletedprojects/04-0747.pdf
USDA-NASS. 2014. Census of Agriculture. USDA- NASS. Available at: https://www.nass.usda.gov/Publications/AgCens us/2012/ (verified 29 March 2019).
Revised Decemberhttps://digitalcommons.usu.edu/extension_curall/2077/ 2019 and published to the Web May 2022
Utah State University Extension
Peer-reviewed fact sheet
Authors
Kyle Egbert, Matt Yost, Bryce Sorensen, Grant Cardon, Niel Allen, and Ryan Larsen
Utah State University Specialists
Related Research






















































