Alfalfa Weevil (Hypera postica G.)

Do You Know?
- Alfalfa weevil has one generation per year and larval populations peak in Utah before or during first cutting (May to June).
- Base management decisions on established weevil action thresholds. Monitor larval populations in early spring using sweep net or stem count methods.
- Insecticide resistance can be reduced by spraying only when needed, properly timing sprays, and rotating between modes of action.
The alfalfa weevil, Hypera postica (Gyllenhal), western strain, was first discovered in the U.S. in 1904 and is now a major pest throughout Utah and the Intermountain West. The Egyptian and Eastern strains were detected in the southwestern and eastern U.S. in 1939 and 1951, respectively. Some have suggested that the Western and Egyptian strains co-occur in southwestern Utah. These strains look very similar but differ in their biology, which leads to differences in management timing.
Although adults and immature weevils (larvae) feed on alfalfa (Medicago sativa L. ) foliage, the larvae are the primary damaging stage. The key to effective alfalfa weevil management is early spring monitoring to determine the management approach and timing before economically significant damage occurs. Natural predators and parasitic wasps are present in Utah, but their effectiveness against alfalfa weevil is highly variable.
While insecticides are the primary management tool used, use them with caution to avoid insecticide resistance and base their use on established alfalfa weevil treatment thresholds.
Identification
Adults are brown, oval beetles, 3/16” long with chewing mouthparts located at the end of a long distinctive “snout" (Fig. 1). Young adults are light brown with a dark brown to black stripe on the thorax and forewings while old adults are often dark brown to black.



Eggs are laid in alfalfa stems in clusters (Fig. 2A). They are small (<1/32”), smooth, oval shaped, and bright yellow when first laid but turn olive yellow as they develop.
Larvae are creamy yellow to light-green with a distinct white line down the center of their backs (Fig. 2B). The head is dark brown to black. Larvae are small ranging from 1/20” upon hatching up to 3/8” as they progress through their four larval stages (instars).
Pupae are found inside larval-spun, brown, capsule-like cocoons (Fig. 2C and 8) and are an inactive transitional stage between the larval and the adult stage.
Life History and Damage
The alfalfa weevil has one generation, or life cycle from egg to adulthood, each year (Fig. 3). Adults overwinter in the field, in leaf litter near field margins, or in natural habitats several miles away. In the spring, shortly after the alfalfa breaks dormancy, adults migrate to the fields and begin feeding. Egg-laying in Utah begins when the alfalfa is 3-4” high. The female chews a hole in the alfalfa stem and lays a cluster of about 10 eggs (Fig. 4). After 4-21 days, the eggs hatch and the larvae climb to the top of the plant to feed on terminal leaves for 2-4 weeks.
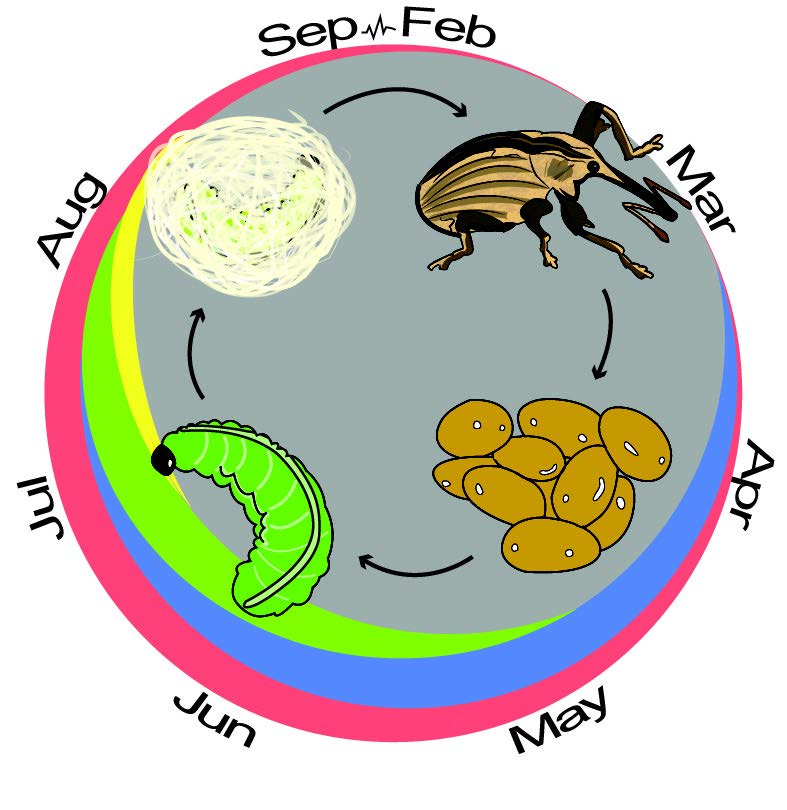

Young larvae are difficult to detect since they feed within protective leaf whorls prior to leaf expansion causing scattered pinholes in leaves (Fig. 5). Older larvae feed exposed between leaf veins on expanded terminal leaves (Fig. 6). As the larvae develop through their four larval stages, each subsequent instar ingests 4-5 times as much foliage as the previous instar. Consequently, most forage loss occurs around the first harvest when larvae are approaching the final larval instar. If development peaks during the last instar (when feeding rates are highest) before first cutting, damage may be severe.
Fully-grown larvae move to the base of the plant or onto the ground to pupate (Fig. 2C). The adult emerges from the cocoon 10-14 days later. New summer adults can feed for several weeks but are unlikely to cause economic damage.
Midsummer, around the second cutting, adults start leaving fields to find winter refuge. Few, if any eggs are laid before winter; however, there is evidence that in some years overwintering eggs may hatch in spring and contribute to larval populations.



locate eggs, look for a dark scar that
develops on the stem around the
damaged area.
Natural Enemies


Since the 1920s, a complex of parasitic wasp species (parasitoids) that attack alfalfa weevils have been introduced to Utah. Only one species, Bathyplectes curculionis (Fig. 7), has become well established. The small dark wasps (1/8” long) deposit an egg into each weevil larva it finds. The weevil larva continues to feed at nearly the same rate as an unparasitized larva while the grub-like parasite larva develops inside. After spinning their cocoon when approaching pupation, parasitized weevil larvae succumb to the parasite, which spins its own pupal case inside the weevil cocoon (Fig. 8). The adult wasps emerge in late-summer to seek out late- developing weevil larvae, or will overwinter in the pupal case to parasitize weevil larvae the following season. Upwards of 70% weevil suppression has been recorded by wasp parasitism; however, parasitism rates are highly variable and not a primary control option.
Predatory insects, including lady beetles, lacewings, and damsel bugs consume weevil larvae, as do many spiders and birds. However, when predators are not abundant early enough to suppress weevil larvae, other control measures may be needed.
Monitoring
Growers are advised to check their fields regularly and spray only when larvae reach economic thresholds. This happens when the financial cost of damage outweighs the treatment cost. Sweep or stem samples should be collected at multiple locations within the field to gather a representative sample for the whole field. Consider collecting from different areas while moving through the field in an X, M, or U pattern.
Sweep net sampling: Use a 15” diameter net to sweep across the top of the alfalfa when it is at least 10" tall. One full sweep is a 180 arc from one side of the body to the other. Use a series of consecutive sweeps (10 sweeps) from multiple areas of the field. Calculate the average number of larvae per sweep by dividing the total larvae collected by the total number of sweeps taken to determine if management is warranted (Table 1).
Table 1. Use sweep net samples to determine when action is necessary.
| Average # larvae per sweep | Action |
|---|---|
| 20 or more | Control warranted |
| 10-19 | Sample again in 3-5 days |
| less than 10 | Sample again in 7 days |
Sweep on calm, clear days (or evenings), with little to no wind. Avoid sweeping when the foliage is damp or wet and be sure to sweep vigorously, but not so vigorously that plants are significantly damaged (or torn off).
Sweeping is particularly effective for late-stage larvae as very young larvae are not easily dislodged from their protected feeding sites inside leaf whorls. As a result, even when young larvae are abundant, sweeping early may appear to inaccurately indicate low weevil abundance. As larvae mature and move to exposed feeding sites, weevil collection rates may rise dramatically, and in some instances may indicate the need for management.
Stem counts: Cut stems at ground level from alfalfa that is at least 10" tall while being careful not to knock larvae off stems. Invert the stems into a bucket (5 gallon) and shake vigorously to dislodge larvae. Measure the average stem height, count the number of stems shaken into the bucket (at least 10 stems per location), and count the number of larvae. Divide the total number of larvae collected by the number of stems sampled to determine management action (Table 2).
Table 2. Use stem count samples to determine when action is necessary.
| Average # larvae per stem | Stem Height | Action |
|---|---|---|
| greater than 2 | 10”-14” | Control warranted |
| greater than 2.5 | 15”-18” | Control warranted |
| greater than 3 | >18” | Control warranted |
| less than 2 | >10” | Sample again in 3-5 days |
| less than 1 | >10” | Sample again in 7 daysSweep |
Terminal leaf damage can be a quick way to determine when sweeping or stem counts are needed. Collect 10 or more leaf terminals at random from the field, count the number of damaged terminals, and determine the percent of terminals damaged ((#damaged/total collected) x 100%). If more than 30% of collected terminals are damaged, sweep- or stem-count sampling is recommended. Terminal leaf sampling is not a replacement for sweep or stem sampling and will not determine if management is warranted.
Temperature Impacts
Temperature is important for insect development and can be an indicator of population growth. Weevil development occurs at or above 48 F. Therefore, during warm springs, weevil damage before the first cutting is common. However, during cool springs when average temperatures are below 48 F, weevil development is delayed and alfalfa is able to “outgrow” damage from weevil larvae that may be still too young at first cutting to cause significant crop damage. Late frosts may delay alfalfa growth, which prevents the crop from “outgrowing” the weevil. Therefore, monitoring fields regularly from green-up to first cutting is recommended.
Environmental Management
Early harvest of alfalfa can be an effective cultural control strategy if larvae do not reach economic levels until the final two weeks before the first cutting. Early cutting can kill young larvae but mature larvae may survive and continue to damage alfalfa regrowth. Therefore, windrows should be bailed as soon as they are dried to 10-20% moisture.
Livestock grazing during winter and spring may help reduce the number of alfalfa weevil eggs.
Interseeding alfalfa with grasses may result in lower weevil populations compared to those in pure alfalfa stands.
Chemical Control
When alfalfa weevil populations reach levels that threaten economic loss, they can be managed effectively with insecticides (Table 3). Many insecticides require a preharvest interval (PHI) of up to three weeks between spraying and cutting to minimize residue contamination. This time period requirement can conflict with cutting schedules so careful consideration is needed before choosing to spray. PHI is found on the label. Insects can develop resistance to insecticide active ingredients (AI) if used frequently. AIs disrupt pest physiology via distinct pathways known as a mode of action (MOA). MOAs are grouped into a classification system with a number (see Table 3). Products with the same mode of action have the same group number. As pests develop resistance to an AI, it can reduce the population's susceptibility to other AIs in the same MOA group or subgroup. For example, early season applications of dieldrin and heptachlor, which are chemically similar and are now banned, to control weevils became ineffective in Utah in the early 1960s.
More recently, pyrethroid-resistant weevils have been detected in California and research is on-going to seek out resistant populations. To reduce insecticide resistance, also rotating among products with different MOA is necessary as rotating between product AIs within the same MOA classification group is not as effective. MOA groups can be found on the Insecticide Resistance Action Commitee's MOA website (https://irac-online.org/modes-of-action) or on the top of the insecticide label. Some insecticides will also kill beneficial pollinators and predatory insects, which may lead to secondary pest outbreaks (such as aphids). Therefore, insecticides should be applied only when weevils reach economic thresholds.
Table 3. This is not an exhaustive list but represents a diverse group of insecticides registered for use against alfalfa weevil. The group number (e.g., 3A) indicates a unique mode of action. Be sure to consult product labels and follow all label requirements before and during application.
| Insecticide | Active Ingredient | Pre-harvest interval | Mode of Action Classification | Remarks |
|---|---|---|---|---|
| Steward EC | indoxacarb | 7 days | 22A | Reasonably safe to bees. Not for seed alfalfa. |
| Warrior II | lambda-cyhalothrin | 7 days | 3A | Do not apply when bees present. |
| Cobalt Advanced | chlorpyrifos, lambda-cyhalothrin | 28 days | 1B, 3A | Extremely hazardous to bees. |
| Entrust SC | spinosad | 3 days | 5 | Can be used in organic growing systems. |
| Baythroid XL | B-cyfluthrin | 7 days | 3A | Extremely hazardous to bees. |
| Lorsban Advanced | chlorpyrifos | 7-21 days | 1B | Will also kill aphids. Do not apply when bees present. |
| Malathion 8-E | malathion | 0 days | 1B | Only apply when 10-15 larvae per sweep at cutting. |
Image Credits
1 & 2B Image courtesy of Erica Stephens, Utah State University
2A, 4, & 6 Image courtesy of Kaitlin Rim, Utah State University
2C Image courtesy of University of Illinois Extension
3, 5, 7, & 8 Image courtesy of Joseph Clarine, Utah State University
Funding for this publication was made possible by the National Institute of Food and Agriculture, U.S. Department of Agriculture: Alfalfa and Forage Research Program (No. 2016-51181-25409), Utah Agricultural Experiment Station, and USU Extension.
Additional Resources
- Insects and nematodes associated with alfalfa in Utah. D.W. Davis, editor. Utah Agricultural Experiment StationBulletin 494 (1976). 59 pp.
- Pellissier, M.E., Nelson, Z., & Jabbour, R. (2017) Ecology and Management of the Alfalfa Weevil (Coleoptera: Curculionidae) in Western United States Alfalfa. https://academic.oup.com/jipm/article/8/1/5/3064074
- Pest Web - Montana. pestweb.montana.edu/Postica/Home/Index
- Shewmaker, G. E., Allen, R. G., & Neibling, W. H. (2013). Alfalfa Irrigation and Drought. uidaho.edu/-/media/UIdaho-Responsive/Files/cals/centers/Kimberly/forage/Alfalfa-Irrigation-Facts-2013.pdf
- Utah State University Extension. (2011). How to Sweep Sample for Pests. youtu.be/hD6VxAQD96k
Published February 2020
Utah State University Extension
Peer-reviewed fact sheet
Authors
Kaitlin Rim, USU Biology; Joseph Clarine, USU Plants, Soil, Climate; Steven Price, Carbon Co. Extension; Ricardo Ramirez, Extension Entomologist
Related Research




















































