Alfalfa Stem Nematode

alfalfa stem nematode. Less than 50% of plants remain
due to die-out from the infected seedlings.
What You Should Know
Alfalfa Stem Nematode (ASN) is a nearly microscopic round worm that enters the alfalfa plant and lives in the stems and leaves, usually above ground. Under ideal conditions (wet weather in late winter or early spring, at 59-70°F), ASN can complete its life cycle from egg to reproducing adult in 19-23 days. A single ASN female, after mating with a male, can produce 200-500 eggs during its reproductive life. ASN can parasitize and persist on a number of host plant species, but can only reproduce in alfalfa and sainfoin. ASN can undergo anhydrobiosis, a state of drying to near death, and persist in plant debris, on seeds, or in dry soil for a very long time.

healthy stem (left) compared with shortened internodes
and swollen nodes on ASN infected stem (right).
Photo courtesy Dr. S.V. Thomson.
Introduction
A lfalfa stem nematode, Ditylenchus dipsaci, belongs to a diverse species of nematode often referred to as stem and bulb nematodes. Within this species are a number of races or strains of the nematode. Alfalfa Stem Nematode is one that attacks and reproduces only on alfalfa (Medicago sativa) and sainfoin (Onobrychis viciifolia) plants. The ASN occurs in all regions of alfalfa production worldwide and can dramatically reduce plant stand and forage yields. Several nematodes are known to parasitize alfalfa; however, ASN is the most serious nematode causing damage in alfalfa production.
ASN is increasingly a concern to alfalfa producers in all regions of Utah; however, noticeable losses have occurred in Millard, Cache, and Box Elder counties. Symptoms are easily recognized in the early spring during cool wet weather. Damage is most often seen in flood-irrigated fields with increased damage observed near the headwater ends of infected fields. Newly established alfalfa on ASN infested ground often declines rapidly with poor seedling stand, increased weed pressure, and poor forage yield. Alfalfa production with moderate to severe ASN pressure (Fig. 1) rapidly becomes unprofitable within a year or two after planting.

infected alfalfa stems.
Symptoms
Infected plants tend to be stunted with very small “mouse eared” leaves. Infected stems have shortened internodes and swollen nodes (Fig. 2). Under the right climatic conditions, infected plants can appear yellow (Fig. 3) or even white in color (often called white flagging). This symptom is readily observed at green-up in the early spring or just after the first cutting. However, chlorotic flagging may be an indicator of other problems and alone does not indicate the presence of the ASN. Crinkled leaves are often observed on infected plants, with crinkling occurring between veins, not crossing them. Infected stems are brittle and tend to break off from the crown. Crowns of infected plants are not firm and may even appear spongy in consistency. Infected areas of the field are about 2-3 weeks slower to green-up in the spring compared to noninfected areas and appear to have winter killed. Although plants persist with moderate ASN pressure, eventually, with increasing ASN populations, they will die and plant stands will become patchy with blank spaces. Stand decline will increase weed pressure.
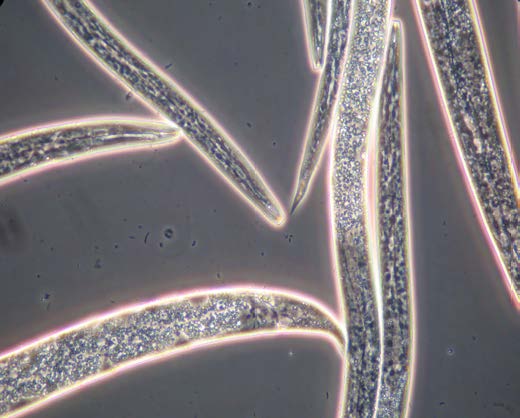
alfalfa stem nematodes.
Disease Cycle
All species of plant parasitic nematodes have a hollow stylet that is used to puncture the plant’s cells so that they can feed on the plant cellular contents. ASN also has this trait (Fig. 4), but is among a small number of nematode species that can live and complete its life cycle, most often, above ground free of soil contact. ASN progresses through several life stages beginning with the egg. Within the egg, the nematode develops and goes through its first molt. After egg-hatch, the nematode goes through two additional molts to become a pre-adult or infective juvenile. At this stage the nematode can withstand extremes of freezing and drying for long periods by persisting in or on the surface of hay or plant debris and/or seed (Figs. 5 and 6), or in the crowns of plants when survival conditions are adverse. When there is sufficient moisture and favorable temperatures, the infective juveniles become active, enter the plant by colonizing seedlings as they germinate or by swimming up on the surface and entering through the plant’s stomates. The nematodes are small enough to move within the plant’s internal open spaces between cells. The nematodes secrete enzymes and plant-affecting hormones as they feed that stunt and swell plant tissues. Within the host plant, the nematodes molt a fourth time to become male and female adults. After mating, females can lay 200-500 eggs during their life. A complete life cycle, from egg to egg-laying adult, is 19-23 days at 59-70 F air temperature. Nematodes escape to the soil when living conditions within the plant become adverse when heavily infected plants can no longer support the nematode’s growing population, or when plants are dying. Pre-adult juveniles can survive in/on plant debris or seed under dry conditions for years (Figs. 5 and 6).

debris in a sample of “brown bag” alfalfa seed.
Diagnosis
To verify that ASN is causing the symptoms in alfalfa, stems and leaves of symptomatic plants can be sampled and checked for the nematodes. Your local county Extension educator may be able to do this for you as they often can provide diagnoses quickly and accurately with the aid of a microscope. If confirmation is desired, then the sample may be sent to the diagnostic laboratory. To do this collect stems with leaves from several plants exhibiting symptoms of infection. Plant tissues should be sealed in a plastic bag and sent to the: Utah Plant Pest Diagnostic Lab, Department of Biology, 5305 Old Main Hill, Logan UT 84322-5349. Care should be taken not to expose the bag to excessive heat or cold so that the specimen and nematodes remain alive until the sample is received. General instructions for sample collection and shipment can be found at: http://utahpests.usu.edu/uppdl/htm/ forms and directing your browser to the diagnostic laboratory sample submission information.
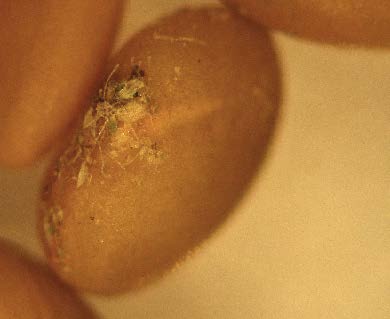
surface of this alfalfa seed from a “brown bag”
source of seed.
Management
Chemical nematicides for controlling ASN are generally ineffective, hazardous, and expensive. Some insecticides are labeled for control of ASN, but studies have shown their efficacy is marginal under ideal conditions at their labeled rates for nematode control. The decision to turn under a field should be based on plant stand and current forage yields rather than nematode population numbers. A comprehensive integrated approach is the best strategy for controlling ASN. Following are recommendations that, if implemented, will reduce the impact of ASN on alfalfa production.
Crop Rotation
Crop rotation is one of the most effective means to control ASN. Planting non-host crops such as small grains (barley or wheat, avoid planting oats), sorghum, or corn for two or more years will reduce stem nematode populations. Longer rotations work better. Special attention should be taken to eliminate volunteer alfalfa during the rotation period, as nematodes will carry over on these plants.
Resistant Varieties
Plant varieties of alfalfa that are resistant to the alfalfa stem nematode, preferably certified seed that carries the official label indicating it is certified. Several varieties are listed as being resistant to the nematode, but measures of resistance can differ. Some companies consider a variety resistant when only 51% of plants test negative for the presence of the nematode under screening trials. Theoretically, this could mean that nearly half the plants will have ASN. Eventually the ASN will overcome resistant plantings and rotation to non-host crops will likely be required. Avoid planting “brown bag” seed (non-certified) that is claimed to be resistant to ASN. It is an unnecessary risk and there are no assurances of seed purity, germination percentage, and the seed may already be contaminated with the nematode (see Fig. 6). No seed certification laboratory, to our knowledge, tests for the presence of the nematode in or on their seed, but certified seed is guaranteed for varietal purity and germination and is sold on that basis. Variety recommendations can be obtained from your local county Extension educator.
Prevention
Pr event nematode reintroduction into a clean field by taking these precautions: cut new clean fields first, be sure the topsoil is dry, do not cut the alfalfa when the top 2-3 inches of soil surface is wet as nematodes will exit the plants and return to the soil once they sense the plant is dying; clean equipment before moving from one field to another; avoid using tail water from a known ASN contaminated field; avoid the temptation to plant “brown bag” seed with claims of pedigree purity and resistance to ASN; avoid the use of manure from cattle operations, where infected hay is used as feed, to spread on rotation crops or new alfalfa plantings as a fertilizer. These recommended “ounces of prevention” are potentially worth tons of forage.
References
- Agrios, G. N. 1997. Plant Pathology, 4th ed. Academic Press, San Diego, CA. pp. 635.
- Summers, C.G. 1998. Integrated Pest Management in Forage Alfalfa. Integrated Pest Management Reviews 3(3):127-154.
- Thorne, G. 1961. Principles of Nematology. McGraw-Hill Book Company, Inc., New York, NY. pp. 553.
Published January 2008
Utah State University Extension
Peer-reviewed fact sheet
Authors
Kent Evans, Extension Plant Pathology Specialist; Clark Israelsen*, Cache County; Mike Pace*, Box Elder County *Utah State University County Extension Agents
Related Research




















































