Take-All Patch
February 2009
Erin Frank, Plant Disease Diagnostician (No longer at USU) • Kent Evans, Extension Plant Pathologist (No longer at USU)
What You Should Know

Take-all patch is a fungal disease of turfgrasses that primarily affects the roots of certain grass species. It has become a major disease of cool-season turfgrasses and can be found in many parts of the United States. The fungus causes a patch disease similar to necrotic ring spot and some of the symptoms of take-all patch can be similar to Microdochium patch. The disease seems to be more severe in areas that were recently cleared of their natural plant inhabitants (native plants such as trees and shrubs), but over time disease severity lessens. Recovery of turf from take-all patch can be slow, but with proper cultural practices disease severity will lessen each year.
Introduction
Take-all patch of turf is a disease caused by the fungus Gaeumannomyces graminis. It causes severe infections in Agrostis species (bentgrass), but also affects Festuca (fescue) and Poa (bluegrass) species. Festuca and Poa species are only occasionally damaged and will normally survive in areas where Agrostis has been killed. Take-all is most severe in areas where natural habitat was disturbed to put in new sod. This is thought to be due to a decrease in the diversity of soil microorganisms that are suppressive to this fungus. Over time the natural buildup of microorganisms that are suppressive to the take-all fungus will reduce the disease incidence and severity. Root infection will occur in the cool months in spring and fall, but above-ground symptoms may not be visible until the plants are stressed by hot, dry weather in the summer.
Symptoms

Symptoms of this disease will typically appear in late spring or early summer. Symptoms begin as small, circular, light brown or reddish brown patches (Figure 1). Symptoms look very similar to Microdochium patch (caused by Microdochium nivale), however, take-all patch will persist into the summer, whereas Microdochium patch symptoms diminish in late spring. Patches can occur in successive years and expand to reach diameters of three feet or more (Figure 2). The edges of the patches or sometimes the entire patch will be reddish brown or bronze in color when the disease is actively developing. During the winter, affected turf may have a gray color. Areas affected by disease will often regrow slowly and the centers of the patches may become invaded by other grass species or weeds. This can cause a “frogeye” symptom similar to turf infected with necrotic ring spot. Symptoms are most noticeable after the plants have been stressed in hot, dry weather in late summer. Mild infections that are not noticeable in spring will develop under conditions like this. In the early stages of infection, dark strands of mycelium will colonize the surface of the roots and grow parallel to the root axis. The roots will develop internal dark brown streaks. As the disease progresses the stolons, rhizomes, roots, and bases of shoots will turn dark brown to black. Once the weather becomes warm and dry the roots will become dark brown and brittle and the plants will be easily pulled from the soil. In late fall small, flask-shaped structures called perithecia will develop on the culms of infected plants.
Disease Cycle
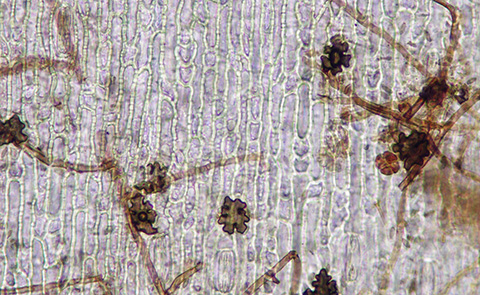

The pathogen will survive unfavorable conditions as perithecia (spore producing fungal structures) embedded in plant tissue and as mycelium in previously infected plant tissue. The ascospores inside the perithecia will penetrate root hairs and root epidermal cells to start an infection. Penetration of the root cells is accomplished by infection structures called hyphopodia, and direct growth of the fungus from large masses of hyphae called mycelial mats. Infection of the stolons, rhizomes, and crowns will occur during the fall and spring. Runner hyphae will colonize the surface of the roots and will give rise to simple hyphopodia (Figure 3) and infection cushions (Figure 4), which will begin infection into the root cortex. As the vascular tissues become plugged with fungal hyphae, dark brown streaks will develop in the root tissue. In cool, moist weather extensive colonization of plant tissues will occur. The initial stages in the development of this disease occur in the early spring and late fall months when the weather is cool and wet. However, severe root damage and plant death may not develop until the hot, dry weather causes plant stress in the summer. Aboveground symptoms will usually appear in late spring or early summer when heat or drought stress will induce decline in infected plants. In severe infections, symptoms may be seen in fall as well as in the spring. The pathogen is most likely spread by direct contact between infected and healthy plants. The fungal hyphae will grow along root surfaces and rhizomes, spreading from plant to plant underground. Long distance movement can occur by the transport of infested soil or plant material, or on contaminated equipment.
Diagnosis
To be sure that take-all is causing the symptoms in turf, samples can be collected and checked for the presence of the pathogen. Your local county extension educator may be able to do this for you as they often can provide diagnoses quickly and accurately. If confirmation is desired, then the sample may be sent to the diagnostic laboratory. To do this, collect samples of turf exhibiting symptoms of infection. Plant tissues should be sealed in a plastic bag and sent to: Utah Plant Pest Diagnostic Lab, Department of Biology, 5305 Old Main Hill, Logan, UT 84322. Care should be taken not to expose the bag to excessive heat or cold so that the specimen and pathogen remain alive until the sample is received. General instructions for sample collection and shipment can be found at: https://extension.usu.edu/pests/uppdl/submission-form and directing your browser to the diagnostic laboratory sample submission information.
Management
Recovery of turf from take-all patch is slow. Planting a mixture of grasses can effectively reduce disease severity. Disease severity can also be reduced by maintaining a soil pH around 5.5 to 6.0. Attempts to lower the soil pH will help, but Utah soils are alkaline (high pH) and this will require an ongoing application of management practices aimed at lowering pH, which can be expensive. Acidifying fertilizers, such as ammonium sulfate, can be applied in cool weather to reduce soil pH over time. It is important to have a balanced fertilization program. Manganese, potassium, and phosphorus deficiencies can enhance the development of the disease. Supplement any deficient sites in the fall or spring to reduce disease severity. Factors that restrict root growth may also increase the severity of take-all patch. Avoid excessive irrigation and nitrogen applications, provide adequate drainage, and aerate turf (using clean equipment) when symptoms are absent to improve air and water movement and alleviate soil compaction. Once symptoms have appeared, maintain adequate irrigation and use fertilizer in frequent, light applications to compensate for damage to the root system.
Resistant Varieties
All species and cultivars of Agrostis (bentgrasses) are susceptible, but there are some cultivars of Agrostis stolonifera available with moderate resistance. Kentucky bluegrass (Poa pratensis) and creeping red fescue (Festuca rubra) are highly resistant.
Prevention
Plants that are severely infected can be easily removed from the soil because of widespread rotting of roots, rhizomes, and crowns. The fungus may be spread by infected sod and mechanical equipment. Effectively cleaning equipment (power steam washer) before working with healthy turf will help prevent the spread of this pathogen. Fungicides are available for controlling take-all, but they must be used as a preventative measure to get the best results. Applications in the fall (before dormancy) and early spring are most effective. Chemical pesticides labeled for the control of take-all include triazoles (Eagle, Banner Maxx, or Bayleton), azoxystrobins (Heritage), fluoxastrobin (Disarm) and pyraclostrobin (Insignia).
References
- Smiley, R.W., Dernoeden, P.H., and Clarke, B.B. 2005. Compendium of Turfgrass Diseases, 3rd ed. APS Press, St. Paul, MN. pp. 91-94.
- Corwin, B., Tisserat, N., and Fresenburg, B. 2007. Integrated Pest Management. Identification and management of turfgrass diseases. University of Missouri Extension. Columbia, MO pp. 40-41.
- Couch, H.B. 1995. Diseases of Turfgrasses, 3rd ed. Krieger Publishing Company, Malabar, FL. pp. 25-28.

