Coryneum or Shothole Blight
May 2008
Kent Evans, Extension Plant Pathology Specialist (No longer at USU) • Erin Frank, USU Plant Disease Diagnostician (No longer at USU) • JayDee Gunnell, Davis County Extension Agent • Mike Pace, Box Elder County Extension Agent • Maggie Shao, Salt Lake County Extension Agent (No longer at USU)

Fig. 1. Bud tissues infected by W. carpophilus are often darker and are sunken with a darkened canker beginning to encircle and radiate from the bud. Eventually the canker will girdle the twig and kill it from that point to the proximal end or tip of the twig or branch.

Fig. 2. Early infection appears as a very darkened bud that is obviously dead. These buds often will exude gum as a sign of the disease. The infected tissue serves as a source of inoculum during continuoulsy moist conditions.

Fig. 3. Infection of new buds at, or during, bud break. Note how the cankered wood is being girdled. The proximal end of the twig will be killed when the canker completely encircles the twig, effectively preventing nutrients and water from being conducted to the uninfected tissues.
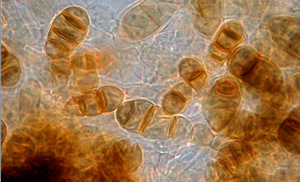
Fig. 4. Pigmented conidia (spores) of Wilsonmyces carpophilus. These spores are very durable and can survive for months exposed to sunlight (high ultraviolet light that often kills hyaline or colorless spores) and extremes of temperature. These spores infect susceptible plant tissues during periods of continuous moisture. Infection periods are determined by duration of moisture conditions and the temperature. At cooler temperatures, longer periods of moisture are required.

Fig. 5. An infected peach leaf exhibits the classic shothole appearance associated with this disease. Infected tissues die and cannot expand as the leaf grows and eventually tear out and fall to the ground leaving an appearance often confused with insect feeding. Infected tissue will often remain connected at one point (see arrow).

Fig. 6. Infection on fruit of apricot (top) and peach (bottom) is common. These spots usually begin as small reddish to purple lesions. The lesions become rough and hard. Corky tissue will develop in the fruit beneath the lesions. These can enlarge and coalesce to produce irregular and larger leathery patches that may crack and show gumming. Though unsightly, the fruit can be usable for canning or consumption with more peeling losses. Infected fruit is not marketable.
What You Should Know
Coryneum blight, also called shothole blight, is a fungal disease of stone fruit trees including peach, nectarine, apricot, cherry, and almond (ornamental as well as nut bearing); however, the most commonly affected are apricot, peach, and nectarine. The fungal pathogen is active during cool, continuously moist, conditions and can infect and cause damage on buds, twigs, leaves, blossoms and fruit of host plants. The fungal pathogen overwinters on infected dormant leaf and blossom buds on twigs. Under sustained moisture, the pathogen can actively colonize host tissues at temperatures as low as 36 °F in as few as 24 hours, while at 77 °F, the fungus can infect a suitable host’s tissues in as few as 6 hours. Combining practices of pruning, to remove infected tissues, and applying chemical fungicides, are very effective measures used to manage this disease.
Introduction
Coryneum blight, also known as shothole blight, is a fungal disease that can cause damage on peach, nectarine, apricot, almonds (ornamental as well as nut bearing), and to a lesser degree, cherries (tart and sweet). Coryneum blight is caused by the fungal pathogen Wilsonmyces carpophilus. Changes in fungal taxonomy explain this pathogen’s name; in the past it was known as Stigmina carpophila or Coryneum beijerinckii. The pathogen can infect buds, twigs and branches, blossoms, leaves, and fruit (Figs. 1-6). The disease is most damaging in extended cool and moist conditions of spring, although this disease can occur and cause damage at anytime during lengthy wet weather.
Disease Cycle
The fungal pathogen overwinters in infected buds (Figs. 1-3) and cankers on infected twigs and branches. Spores produced from these infected tissues, in the early spring, are dispersed during rain events to infect new buds (Fig. 3). Later in the season, other susceptible tissues can become infected when there is suitable moisture on leaves and fruit. The spores (Fig. 4), called conidia, are pigmented and are extremely durable. They can survive, exposed in a dormant state, on the surface of a bud for months, waiting for just the right temperature and moisture conditions to germinate and infect its host. This pathogen often surprises growers as it is active in the early spring at cold temperatures. The fungal pathogen can infect a suitable host if moisture is continuous for 24 hours or longer at 36 °F, meaning that infections can occur when host plants are still dormant. At higher temperatures, W. carpophilus needs only 6 hours to infect a host plant under continuous moisture at 77 °F. Coryneum blight can develop rapidly under warmer temperatures, and will sporulate from infected tissues through the season during extended wet conditions.
Symptoms
Infections on leaves (Fig. 5) will develop small round purple to tan lesions that are seldom 1/4 of an inch in diameter. Infected tissues can become raised and scurfy and will often drop out as the diseased tissue cannot expand with the growing leaf. Lesions can be circular to slightly elipsoid. These diseased leaf tissues will tear along the lesion margins and may hang on at one attached point, but eventually drop out giving the shothole appearance. Infected buds will often develop a canker that can expand to girdle the twig and kill it. Often infected buds will show signs of gumming. These infected buds are easily recognized as they are often darker than healthy noninfected buds. Infection on fruit (Fig. 6) often appears first as small purple spots that become white to gray lesions, often accompanied by gumming. Infections on fruit degrades their quality and often will result in the loss of the fruit.
Management
Cultural control practices involve removing infected twigs and branches by pruning and destroying wood. Thorough pruning during the dormant season is very effective and recommended for the homeowner as a major component of management to control this disease. In the spring at shuck fall, fungicides such as Abound, Pristine, Gem, Echo 720, Bravo Weather Stik, and Ziram, are effective (see Table 1). For commercial growers, protective fungicides should be used during frequent wet weather events. During watering events, avoid wetting of branches, twigs, leaves, and fruit. In the fall, chemical controls to apply at 50% leaf drop include copper compounds such as Bordeaux mixture, copper based products like Kocide, and fungicides such as Ziram. Chemical applications in the fall will help to protect buds during the dormant season. With regard to any chemicals mentioned, read the label and follow the labeled instructions for their use. Mention or exclusion of trade names in this publication does not represent or imply endorsement nor criticism of any chemical product. See Table 1 for more details regarding chemical controls.
Diagnosis
Most of the diagnostic symptomology has already been introduced in previous sections of this fact sheet. However, if a person wishes, they can take a sample twig, branch, leaves, or fruit to their local county Extension agent. The agent can often diagnose this disease on sight and provide the client with recommendations. If there are any questions, the Extension agent may also recommend samples of suspect plant tissue or fruit be sent to the Utah Plant Pest Diagnostic Laboratory (UPPDL). Directions for collecting and submitting samples of plant material to the UPPDL can be found online at http://utahpests.usu.edu/uppdl/htm/forms. There are forms available for both insect and plant specimen submissions. Follow the instructions to properly collect and submit samples for diagnoses.
Table 1. Chemicals recommended for the management of Coryneum or shothole blight of stone fruits may not be sold in volumes that are convenient to the homeowner; in higher volumes these can be rather inconvenient and expensive. Chemicals are listed, primarily, to inform a homeowner so they can effectively communicate with a licensed chemical applicator. Labeled chemical recommendations often change from one season to the next, always read and be familiar with labeled instructions for the use of the chemical. Specimen labels for these, and many other, compounds are available at www.cdms.net on the Internet. Another reference is the Utah Home Orchard Pest Management Guide (https://extension. usu.edu/files/publications/factsheet/home-orchard-guide08.pdf). Specific mention or exclusion of Web sites, their address, or their content represents neither endorsement nor criticism of any that are available for view on the Internet. If you have questions, contact your county Extension office.
| Chemical | Active Ingredient(s) | Rate (always read the label) | Timing of application |
|---|---|---|---|
| Abound1 | Azoxystrobin | 11-15 fl. oz./acre/season | See label. |
| Pristine | Pyraclostrobin + Boscalid | 14.5 oz./acre (do not exceed 5 applications per season, see label) |
See label. |
| Gem | Trifloxystrobin | 6-8 oz./acre | See label. |
| Bravo Weather Stik | Chlorothalonil | 3.125 to 4.125 pints/acre | See label. Fall application |
| Ziram 76DF | Ziram (a zinc compound, see label) |
See label, varies by crop. | See label. Varies by crop. |
| HI-YIELD Bordeaux mix fungicide2 | copper sulfate + lime (a mixture of 8 lb copper sulfate + 8 lb lime/100 gal water) |
See label. | See label. Fall application |
| Kocide 20002 | copper hydroxide | See label, varies by crop. | See label. Fall application |
| Basic copper 532 | basic cupric sulfate | See label. | See label. Fall application |
| C-O-C-S WDG2 | basic cupric sulfate + copper oxychloride |
See label. | See label. Fall application |
1Avoid spraying apples with Abound, it is extremely phytotoxic to some cultivars and can cause excessive leaf drop/defoliation.
2Pay special attention to labeled directions regarding when copper compounds can be used relative to tree physiological stage of development. Copper compounds can cause phytotoxicity, leaf scorching, and fruit russeting if used improperly. Fall application of these compounds is less likely to cause damage as trees are approaching dormancy and fruit have already been harvested.;
References
- Ogawa, J.M., Zehr, E.I., Bird, G.W., Ritchie, D.F., Uriu, K., and Uyemoto, J.K. 1995. Compendium of stone fruit diseases. American Phytopathological Society, St. Paul, MN.
- Thomson, S.V., and Ockey, S.C. 1998. Coryneum blight of stone fruits. Utah State University Extension Fact Sheet. Plant Disease Control No. 25a.
Funding
Funding for this publication was made possible by the National Institute of Food and Agriculture, U.S. Department of Agriculture, Specialty Crop Research Initiative (under award number 2016- 51181-25409), U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Plant Protection and Quarantine (USDA-APHIS-PPQ) (under award number 201699-00001), USU Extension, and the Utah Agricultural Experiment Station.
Related Research











