Quagga Mussel (Dreissena bugensis) and Zebra Mussel (Dreissena polymorpha)
June 2021
Ann Mull, Extension Assistant (No longer at USU) • Lori Spears, Extension Entomologist (No longer at USU)
 Fig. 1 Zebra mussels (D. polymorpha) on beach. Image courtesy of US Environmental Protection Agency - Great Lakes National Program Office, USEPA, Bugwood.org.
Fig. 1 Zebra mussels (D. polymorpha) on beach. Image courtesy of US Environmental Protection Agency - Great Lakes National Program Office, USEPA, Bugwood.org.
Fig. 2 Quagga mussel (D. bugensis) adults. Image courtesy of Michael Massimi, Barataria-Terrebonne National Estuary Program, Bugwood.org.
 Fig. 3 Zebra mussel (D. polymorpha) adults. Image courtesy of National Park Service, nps.gov/lake/learn/quagga-mussel.htm.
Fig. 3 Zebra mussel (D. polymorpha) adults. Image courtesy of National Park Service, nps.gov/lake/learn/quagga-mussel.htm.Quick Facts
- Zebra mussels and quagga mussels are invasive freshwater mollusks native to Eurasia that pose a significant threat to Western aquatic ecosystems.
- Most adults are about the size of an adult human's thumbnail.
- Once these invasive mussels become established, they are difficult to eradicate and require expensive, ongoing maintenance.
- Invasive mussels are highly efficient at filtering water which results in depleted food sources for native species and explosive growth of bottom algae and weeds.
- Infested areas can have devastating impacts on native mussel populations, fisheries, hydropower operations, and municipal water utilities.
- The quagga mussel was first detected in Lake Powell in 2012 and quickly infested the reservoir.
- The zebra mussel is NOT known to occur in Utah, but living specimens were found hitchhiking in imported aquarium “moss balls” sold in Utah and in pet stores nationwide in 2021.
The quagga mussel and the zebra mussel (Order: Veneroida, Family: Bivalvia) (ZQM) (Figs. 1-3) are two-shelled mollusks native to the Caspian and Black Seas in eastern Europe and western Asia that spread rapidly and can severely disrupt aquatic ecosystems and critical water infrastructure systems. In North America, these invasive dreissenid mussels were first discovered in the Great Lakes in the late 1980s from discharged contaminated ballast water from ships arriving from Europe or through mature adults attached on anchors stored in the internal compartments of these ships. Within three years, they had been detected in all five Great Lakes. Currently, the Columbia River Basin is one of the few remaining areas in the U.S. that is free from these invasive mussels.
The quagga mussel now occurs in many eastern and midwestern states as well as in select waterbodies in the western states of Arizona, California, Colorado, New Mexico, Nevada, and Utah. In the western U.S., this species was first detected in the lower Colorado River in 2007 in Lake Mead, Lake Mohave, and Lake Havasu, 1,000 miles west of the nearest known colony at that time, likely having been introduced from contaminated trailered watercraft. About a year later, it was found in numerous lakes and reservoirs in California, Colorado, and New Mexico, and in 2012 it was detected in Utah in Lake Powell, where it quickly infested the reservoir. Immature quagga mussels were detected in Utah in Sand Hollow and Deer Creek Reservoirs in 2012 and 2015, respectively, but subsequent sampling of plankton tows yielded no invasive mussels, and these waterbodies are currently considered free of invasive mussels (R. Gibbs, Utah Department of Wildlife Resources aquatic invasive species biologist, personal communication, March 12, 2021).
The zebra mussel now occurs in all large navigable rivers in the eastern U.S. and has been detected from hundreds of inland lakes in 28 states, including Texas, Montana, Colorado, and California (Benson et al., 2021). In Utah, immature zebra mussels were detected in Emery County in 2008 in Electric Lake and Red Fleet Reservoir. The Utah DWR subsequently conducted and analyzed plankton tows for years following these initial detections and found no invasive mussels. This suggests that environmental conditions were unfavorable for development of these species. These waterbodies are currently considered to not have invasive mussels (R. Gibbs, Utah Department of Wildlife Resources aquatic invasive species biologist, personal communication, March 12, 2021).
Description
ZQM adults are small, up to about 1.5 inches (40 mm) in length with two triangular shells and byssal threads (ropes) on the hinged edge, which are not found on native mussels (Figs. 4 and 5). Note that these threads may get pulled out when the mussel is removed from the substrate (stopaquatichitchhikers.org 2017). Color patterns can vary greatly in both species, and stripes on shells may be bold, faint, horizontal, vertical, or absent. In areas of species overlap, quagga mussels can more closely resemble zebra mussels and may require genetic identification (Benson et al., 2021, CABI, 2019a). Interbreeding of the two species is considered unlikely under natural conditions (CABI, 2019a).
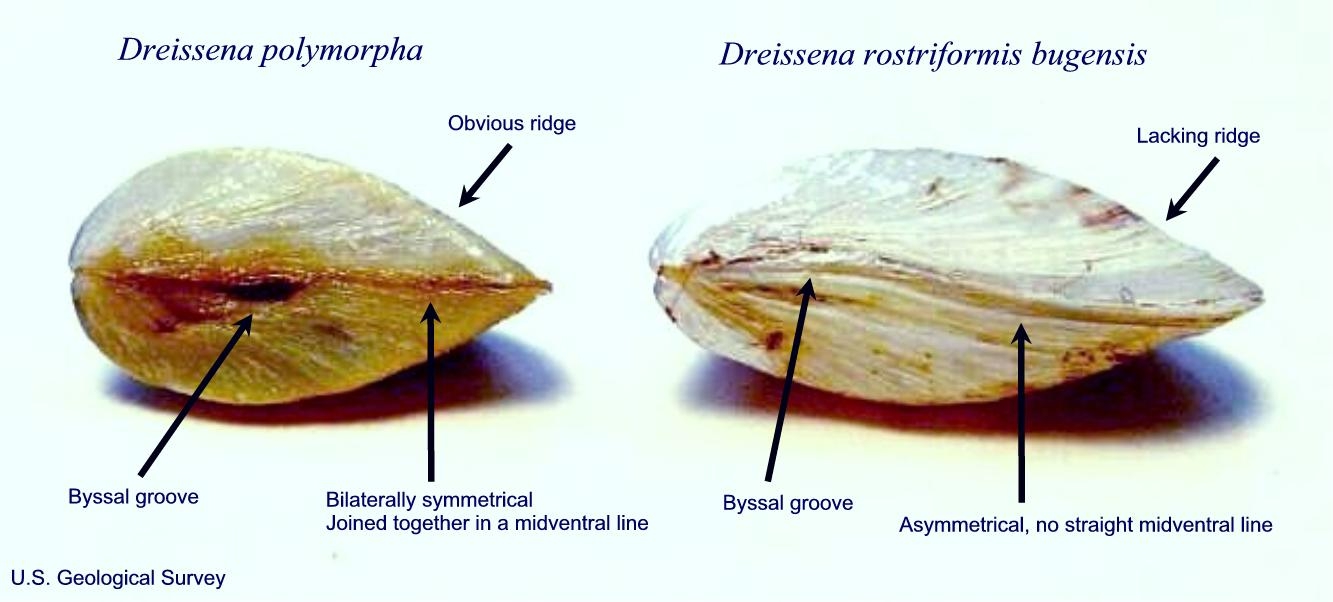 Fig. 4 Zebra mussel (left) and quagga mussel (right). Image courtesy of Myriah Richerson, US Geological Survey.
Fig. 4 Zebra mussel (left) and quagga mussel (right). Image courtesy of Myriah Richerson, US Geological Survey. Fig. 5 Byssal threads on a dreissenid mussel. The threads do not occur on native mussels. Image courtesy of Minnesota Aquatic Invasive Species Research Center.
Fig. 5 Byssal threads on a dreissenid mussel. The threads do not occur on native mussels. Image courtesy of Minnesota Aquatic Invasive Species Research Center.Quagga Mussel
The quagga mussel adult has a more rounded appearance than the zebra mussel. Colors vary widely and range from brownish-yellow to completely black, including intermediate forms that have stripes of varying shapes and sizes (Figs. 2, 6, 7). The two shell halves are asymmetrical and form a curved line where they meet (Fig. 4). The ventral (hinge) surface is convex. The thickest portion of the shell is pointed, and a distinguishable white stripe or line that crosses the middle of the shell from the umbo (most prominent, highest part of the shell) toward the posterior end may be visible. A distinct form of quagga mussel that is pale or all white in color occurs in the deep waters of Lake Erie (CABI 2019a, USFWS n.d.).

Fig. 6 A quagga mussel adult. Image courtesy of Amy Benson, U.S. Geological Survey, Bugwood.org.

Fig. 7 Quagga mussel adults can
have a more rounded appearance. Image courtesy of Amy Benson, U.S. Geological Survey, Bugwood.org.
Zebra Mussel
The zebra mussel adult has a more flattened appearance than the quagga mussel. The two shells are symmetrical in shape and meet to form a straight midventral line (Fig. 4). The flattened underside enables it to remain upright when placed on a flat surface and is often a distinguishing characteristic (Figs. 3, 8). Colors typically range from black or brown with variable white to yellow zebra-like stripes or zigzag patterns (Fig. 8) (Benson et al., 2021, CABI, 2019b).

Fig. 8 Zebra mussel adults have a more flattened appearance in general. Shells can vary widely in pattern and coloration. Image courtesy of Amy Benson, U.S. Geological Survey, Bugwood.org.
Life History
ZQMs have three life stages: larval, juvenile, and adult. Adult ZQMs release eggs into fresh or waterbodies such as lakes, reservoirs, rivers, ponds, and quarries in spring to summer. Within 2 days, the eggs hatch into microscopic free-swimming veligers (larvae) that move passively downstream on flowing water for up to a month and eventually attach onto surfaces such as rocks, docks, cement, wood, plastic, metal, rope, and boat hulls. Once anchored, they undergo metamorphosis within 3 to 5 days at 72°F (22 °C) and emerge as juveniles that will grow into adults in 3 to 5 weeks (Benson et al., 2021, CABI, 2019a, McMahon, 2012).
Each adult can filter about 1 liter of water daily as it feeds primarily on algae and phytoplankton. ZQMs become sexually mature in the first or second year, and each female can produce over 40,000 eggs per reproductive cycle and over one million eggs per spawning season (Benson et al., 2021, CABI, 2019a). Adults typically live for 2 to 5 years. Adults and immatures (eggs, larvae) can be introduced to new areas passively from flowing water or as hitchhikers on host material such as watercraft and aquatic plant material sourced from infested waters.
Zebra mussels prefer 68 to 77 °F waters (20 to 25 °C) but can persist in temperatures up to 86 °F (30 °C). They reproduce in water above 54 °F (12 °C) (CABI, 2019b). In areas of overlap, quagga mussels can outcompete zebra mussels, although zebra mussels remain dominant in inland lakes and rivers (Benson et al., 2021, Hoddle, n.d.). In a laboratory study, ZQM exposed to 5% or greater water salinity resulted in total mortality within 18 days (Spidle et al., 2011).
Quagga mussels tolerate cold waters better than zebra mussels and can survive temperatures between 33 and 86 °F (1 to 30 °C). They reproduce in waters above 48 °F (9 °C). In Lake Mead, they reproduce year-round, and largely settle on surfaces that are from about 20 to 92 feet (6 to 28 m) deep. The highest settlement rates of veligers occur from mid-October to mid-December (Wong et al., 2012). In Lake Michigan, they filter feed year-round and have been found as deep as 540 feet (Hoddle, n.d.).
Impacts
ZQM can cause significant ecological, economical, and recreational impacts (Figs. 9 to 12). They filter and clean the water at unprecedented rates, resulting in depleted food sources (phytoplankton) for native fish and invertebrates, and increased sunlight into waters that can cause explosive growth of bottom algae and nuisance weeds that choke native vegetation and get washed ashore to rot. In established areas, they have been associated with damaging outbreaks of avian botulism and have caused amphipod and fish populations to crash (Hoddle, n.d.). They can suffocate native mussels by anchoring onto them by the thousands and have caused a severe decline of native mussels (Benson et al., 2021). ZQM can be a nuisance by clogging boat motors and requiring mandatory, and at times lengthy, boat inspections, and they can pose health issues when walking on beaches covered with the sharp-edged mussels that appear as waters recede. They can affect irrigated agricultural land by infesting canals and pipelines and clog irrigation pumps, screens, and head gates, and reduce pumping capacity. ZQM can rapidly clog pipes and other water inlets and outlets, and can cost the water industry up to $1 billion annually for management, which typically entails physically dislodging them from surfaces. In Utah, quagga mussel management at Lake Powell from 2000 to 2013 cost over $7.5 million (NPS, 2018). A possible short-term benefit of ZQM is that water clarity in polluted lakes may benefit by their intensive filtration (Hoddle, n.d.).

Fig. 9 Zebra mussels on a native freshwater mussel. Image courtesy of Ohio Sea Grant, Ohioseagrant.osu.edu.

Fig. 10 Quagga mussels exposed by lower water levels at Glen Canyon National Recreation Area. Image courtesy of David Rankin, National Park Service.

Fig. 11 Zebra mussels on grocery cart after only a few months submerged in infested waters. Image courtesy of James F. Lubner, University of Wisconsin Sea Grant Institute.

Fig. 12. Routine cleaning of quagga mussels from penstock gates at Glen Canyon Power Plant. Image courtesy of Chris Watt, U.S. Bureau of Reclamation.
Zebra Mussels in Aquarium Moss Balls
In March 2021, zebra mussels were discovered in a variety of algal “moss balls” (a.k.a. Marimo Balls) sold at aquarium and pet-supply stores in at least 42 U.S. states from Alaska to Florida, including Utah, as well as in British Columbia, Canada (Figs. 13 and 14). The moss ball is a species of green algae that is formed into a 2- to 5-inch diameter ball for use in home aquariums. The USFWS,, the Pet Industry Joint Advisory Council (PIJAC), and the Utah Division of Wildlife Resources (DWR) are urging pet and aquarium stores and aquarium owners to treat all purchased moss balls as though they are infested with zebra mussels and to properly destroy these materials to mitigate the risk of introduction. Decontamination is highly recommended and is essential if mussels are observed. Keep in mind the veliger stage is microscopic and therefore not easily observed (PIJAC, 2021, USGS, 2021).
Destroy the moss ball using one of the following methods: Freeze it by placing it in a sealable plastic bag in the freezer for a minimum of 24 hours; boil it for a minimum of 1 minute; or submerge in regular, unscented bleach diluted to 1/3 cup per gallon of water for 10 minutes or in undiluted white vinegar for 20 minutes. Once the moss ball is destroyed, place it and all its packaging in a sealed bag or container and place in the trash. If boiling water, vinegar, or bleach was used, the liquid can be poured down a household drain. Do not pour any liquid into a storm drain. Most importantly, DO NOT flush the moss balls, mussels, aquarium water, or other suspect materials down the toilet and DO NOT dump them outside (USFWS, 2021a).
To decontaminate the aquarium that housed the moss ball(s), place fish and other living organisms in a separate container with water from an uncontaminated source, then sterilize the contaminated aquarium water by pouring it into a separate container, adding 1/3 cup bleach for each gallon of water, and allow it to sit for 10 minutes before pouring down a household drain.
Clean aquarium substrate, rocks, décor, and filter media by using one of the following methods that is in accordance with the manufacture's recommendations:
- Hot Water Method. Flush and coat the tank and all accessory surfaces with 120 to 140 °F water for a minimum of 2 minutes.
- Salt Water Method. Soak aquarium accessories in a saline solution of ½ cup of salt per gallon of water for a minimum of 24 hours. When finished, pour the treated water into a household drain and rinse all items well before setting up the aquarium.
- Bleach Disinfection Method. Soak aquarium accessories in a solution of 1/3 cup bleach per gallon of water for 15 minutes. Replace the filter media with new media. Ensure all items are rinsed completely prior to setting up the aquarium, and use a dechlorinating product to neutralize any residual chlorine before reintroducing fish and other living organisms (USFWS, 2021a, PIJAC, 2021).
Under certain situations, quarantining your aquarium may be an option. If mussels have not been observed, closely inspect the aquarium weekly for a minimum of 6 months. If you detect the presence of mussels, follow the decontamination steps listed above, and decontaminate and dispose of all live plant material. The aquarium and its contents must be decontaminated prior to resuming selling or trading. The quarantine period ends after 6 months of mussel-free observations (USFWS, 2021b).
 Fig. 13. A moss ball sold in pest stores that contains an invasive zebra mussel. Image courtesy of Center for Invasive Species and Ecosystem Health.
Fig. 13. A moss ball sold in pest stores that contains an invasive zebra mussel. Image courtesy of Center for Invasive Species and Ecosystem Health.
Fig. 14. Zebra mussels on imported
moss balls. Image courtesy of U.S. Geological Survey.
Monitoring
ZQMs are often so tiny than human inspectors can miss them, but performing self-checks and stopping at all mandatory inspection stations when transporting watercraft remain critical steps in stopping their spread. Many incidents of mussels being detected in their second or more inspection stop have been reported. When boating, inspect all watercraft and associated accessories (e.g., dry wells, gear, anchors, lines, and swim ladders) before and after each outing. Also inspect Jet Skis, Waverunners, canoes, kayaks, float tubes, float planes, paddleboards, all SCUBA gear, and all fishing gear, including bait buckets which can transport larvae in the water. Remove debris, and drain and dry all areas. Leave the drain plugs removed during your trip home. Be aware that ZQMs can live for several days or longer outside of water (Benson et al., 2021).
Hundreds of water samples from Lake Powell are analyzed each year to determine the range and extent of mussel colonization, and these samples are analyzed by methods that include automated particle analysis (FlowCAM) and polymerase chain reaction (PCR) testing which amplifies DNA and enables detection at the planktonic stage (NPS, 2019, USFWS, n.d.). New approaches to monitoring ZQM are bring used in California and Washington State, where dogs trained to detect invasive mussels are being employed (Bureau of Reclamation, 2020, WDFW, 2020), and in Utah, where the DWR has plans to begin decontaminating boats at Lake Powell using a dip tank.
Management
In the Western U.S., only some states have a ZQM prevention program in place, which poses a serious threat to all western waterbodies. No ecologically sound, wide-scale treatment or technology exists to eradicate or control established ZQM populations, but the spread of mussels is preventable (NPS, 2018; Western Regional Plan on Aquatic Nuisance Species, 2020). In Utah, Glen Canyon National Recreation Area coordinates mussel prevention and containment programs at Lake Powell with the help of about 20 local, state, and federal organizations that include the National Park Service, Bureau of Reclamation, and Utah Division of Wildlife Resources (NPS, 2018).
Cultural Control
Current control methods involve inspections and physically removing the mussels by methods such as scraping, scrubbing, high-pressure water jetting, and hot water sprays. Boaters and other users should clean, drain, and dry boats and other at-risk objects (NPS, 2018).
Biological Control
Although many species are known to be natural enemies of ZQM, reports of their ability to significantly suppress mussel populations are rare and concern only local areas. In North America, ZQMs have few natural predators. In the Great Lakes region, lake sturgeon and blue crab feed on these mussels. Known fish predators are species native to the eastern U.S. and include freshwater drum (Aplodinotus grunniens), round goby (Neogobius melanostomus), and redear sunfish (Lepomis microlophus) (CABI, 2019a). In an infested lake enclosure in the southwestern U.S., introduced redear sunfish stocked at high density levels were efficient at removing adult quagga mussels, but the mussels were not eradicated (Wong et al., 2013). Documented bird predators include greater scaup (Aythya marila), lesser scaup (Aythya affinis), and goldeneye (Bucephala clangula) (CABI, 2019a).
Chemical Control
Oxidizing biocides such as chlorine, bromine, hydrogen peroxide, ozone, and potassium permanganate have been used for control under certain situations. Copper sulfate and copper carbonates or chelates can be used in open water systems but require special permits (CABI, 2019a, Hoddle, n.d.).
References and Further Reading
- Benson, A. J., Raikow, D., Larson, J., Fusaro, A., Bogdanoff, A. K., & Elgin, A. (2021). Dreissena polymorpha (Pallas, 1771) [Fact sheet]. U.S. Geological Survey, Nonindigenous Aquatic Species Database.
- Bureau of Reclamation. (2020, June 4). Reclamation announces mussel-sniffing dogs to protect New Melones Lake from invasive species [Press release]. Bureau of Reclamation, U.S. Department of the Interior.
- CABI. (2019a). Dreissena rostriformis bugensis (quagga mussel). Invasive Species Compendium, CAB International.
- CABI. (2019b). Dreissena polymorpha (zebra mussel). Invasive Species Compendium, CAB International.
- Hoddle, M. (n.d.). Quagga & zebra mussels. Center for Invasive Species Research, University of California at Riverside.
- McMahon, R. (2012). Quagga/zebra mussel life history. AG/AIS Workshop, Department of Biology, University of Texas at Arlington.
- National Park Service (NPS). (2018). Glen Canyon: Mussel frequently asked questions, updated March 14, 2018. U.S. Department of the Interior.
- National Park Service (NPS). (2019). Mussel monitoring, updated March 26, 2019. U.S. Department of the Interior.
- Pet Industry Joint Advisory Council (PIJAC). (2021). Enhanced guidance to eliminate zebra mussel invasive species threat [Press release].
- Spidle, A. P., May, B., & Mills, E. L. (2011). Limits to tolerance of temperature and salinity in the quagga mussel (Dreissena bugensis) and zebra mussel (Dreissena polymorpha). Canadian Journal of Fisheries and Aquatic Sciences, 52, 2108–2119.
- Stopaquatichitchhikers! (2017). Protect your waters!
- U.S. Fish and Wildlife Service (USFWS). (n.d.). Zebra mussel (Dreissena polymorpha) quagga mussel (Dreissena bugensis). U.S. Fish and Wildlife Service.
- U.S. Fish and Wildlife Service (USFWS). (2021a). Destroy! Don’t dump! U.S. Fish and Wildlife Service.
- U.S. Fish and Wildlife Service (USFWS). (2021b). Invasive species alert! Updated March 17, 2021. Habitattitude.
- U.S. Geological Survey (USGS). (2021). Invasive zebra mussels found in pet stores in 21 states [Press release]. U.S. Geological Survey.
- Western Regional Panel on Aquatic Nuisance Species. (2020, September 29). Updated recommendations for the quagga and zebra mussel action plan for Western U.S. waters. Western Regional Panel on Aquatic Nuisance Species.
- Wong, W. H., Gerstenberger, S., Baldwin, W. & Moore, B. (2012). Settlement and growth of quagga mussels (Dreissena rostriformis bugensis Andrusov, 1897) in Lake Mead, Nevada-Arizona, USA. Aquatic Invasions. Proceedings of the 17th International Conference on Aquatic Invasive Species, San Diego, USA, 29 August-2 September 2010, 7, 7–19.
- Wong W. H., Gerstenberger, S. L., Hatcher, M. D., Thompson, D. R., & Schrimsher, D. (2013). Invasive quagga mussels can be attenuated by redear sunfish (Lepomis microophus) in the Southwestern United States. Biological Control, 64, 276–282.