Asian Longhorned Beetle
May 2022
Ann Mull, Extension Assistant (No longer at USU) and Lori R. Spears, CAPS Coordinator (No longer at USU)
Quick Facts
- Asian longhorned beetle (ALB) is a large wood-boring pest that threatens maple and other North American hardwood tree species.
- The larval stage is the most damaging. Trees infested with larvae do not recover, and larval feeding damage can decrease property values in residential areas.
- ALB has no known natural predators in North America.
- Citizen reports of ALB sightings have aided eradication efforts in every state where this pest has occurred.

Introduction
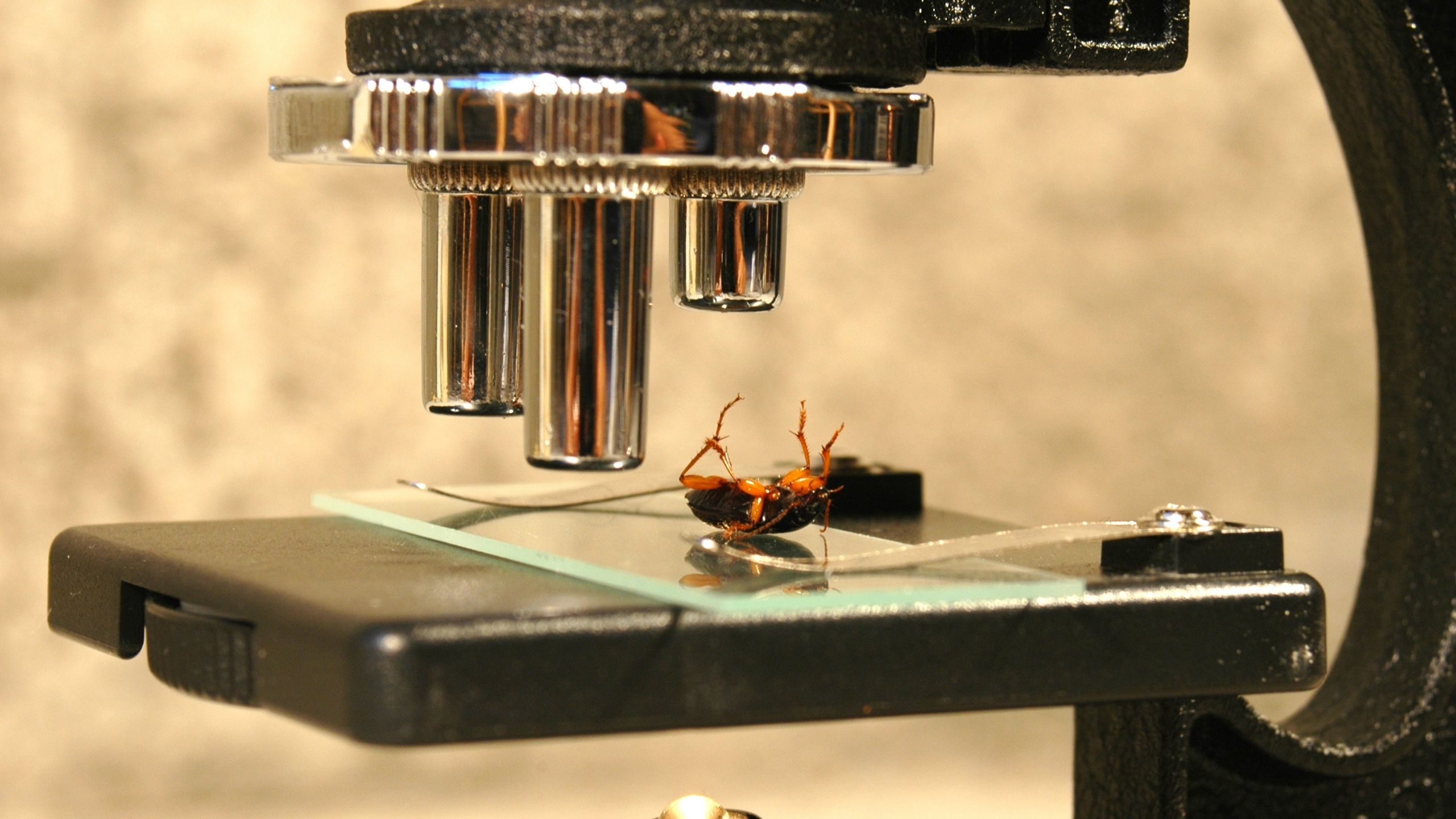
Asian longhorned beetle (Coleoptera: Cerambycidae) (ALB) (Fig. 1) is an invasive wood-boring pest that threatens maple and other hardwood tree species, fall-foliage tourism, and maple syrup production in North America. ALB is also known as starry sky beetle and is native to China and the Korean Peninsula where it is a pest of poplars (Populus spp.) and willow (Salix spp.). ALB was initially detected in North America in 1996 (Brooklyn, New York), likely having arrived on untreated wood packing crates that originated in China. ALB is present in New York, Massachusetts, Ohio, and South Carolina, where eradication efforts are currently underway (Animal and Plant Health Inspection Service [APHIS], 2021). Federal and state quarantines exist to limit the spread of this pest. If you suspect ALB in Utah, please contact the Utah Department of Agriculture and Food (UDAF) or the Utah Plant Pest Diagnostic Lab (UPPDL).
Description
Adults (Figs. 1 and 2) are showy, conspicuous, large, shiny black beetles that are 3/4 to 1 1/2 inches (2 to 3.8 cm) in length with about 20 irregular whitish spots on the glossy-smooth black wing covers (elytra). Notably, the scutellum (central black triangular segment near the upper wing area) is black (Fig. 2). Each antenna has 11 segments, each with a white or whitish-blue base, and is from 1.3 (females) to 2.5 times (males) the body length. The legs, feet, and antennae of newly emerged ALB can have a blueish hue

Other Life Stages
Eggs (Fig. 3) are 1/4 inch (6 mm) long, oblong, and resemble a rice grain with slightly concave ends. Eggs are off-white in color but turn yellowish-brown just prior to hatching.
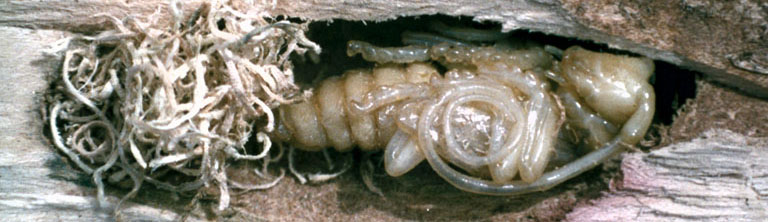
Larvae (Fig. 4) are legless, creamy-white grubs with mandibles that protrude from an otherwise inconspicuous head. Mature larvae are about 2 inches (5 cm) long.
Pupae (Fig. 5) are off-white and about 1 1/4 inch long (3.2 cm) and 1/2 inch (1.3 cm) wide, with a protruding eighth abdominal segment.


Plant Hosts
In North America, ALB mainly attacks maple (Acer spp.), but hosts include all tree species within the 12 genera listed below (APHIS, 2020).
- Maple (Acer)
- Ash (Fraxinus)
- Poplar (Populus), including quaking aspen (P. tremuloides)
- Elm (Ulmus)
- Birch (Betula)
- Willow (Salix)
- Mountain ash (Sorbus)
- Sycamore (Platanus)
- Horsechestnut/buckeye (Aesculus)
- Mimosa (Albizia)
- Golden raintree (Koelreuteria)
- Katsura (Cercidiphyllum)
In a host-preference study, sugar maple (A. saccharum, from which maple syrup is derived) had significantly higher egg-laying (oviposition) sites and living larvae than red maple (A. rubrum), green ash (F. pennsylvanica), or red oak (Q. rubra) (Morewood et al., 2003). ALB has not been found on conifers, beech (Fagus spp.), and oak (Quercus spp.), although Q. rubra is capable of sustaining egg and larval development (CABI, 2021). This pest is thought to be less of a threat in the western U.S. due to the lack of suitable host plants outside urban areas, but the potential for serious altering of ecological diversity and wetland areas exists if ALB spreads into natural forests (Global Invasive Species Database, 2022).
Damage Symptoms
Symptoms begin after 3 to 4 years of infestation and include weeping sap emanating from egg-laying sites, branch dieback, unseasonable yellowing leaves, and dropped or dying branches. Repeated damage (Fig. 6) results in tree crown dieback and eventual tree death. Trees typically die in 7 to 15 years depending on health and site conditions, and infested trees neither recover nor regenerate (APHIS, 2020). Infested trees are removed to prevent ALB from spreading, which can affect community aesthetics (Fig. 7)
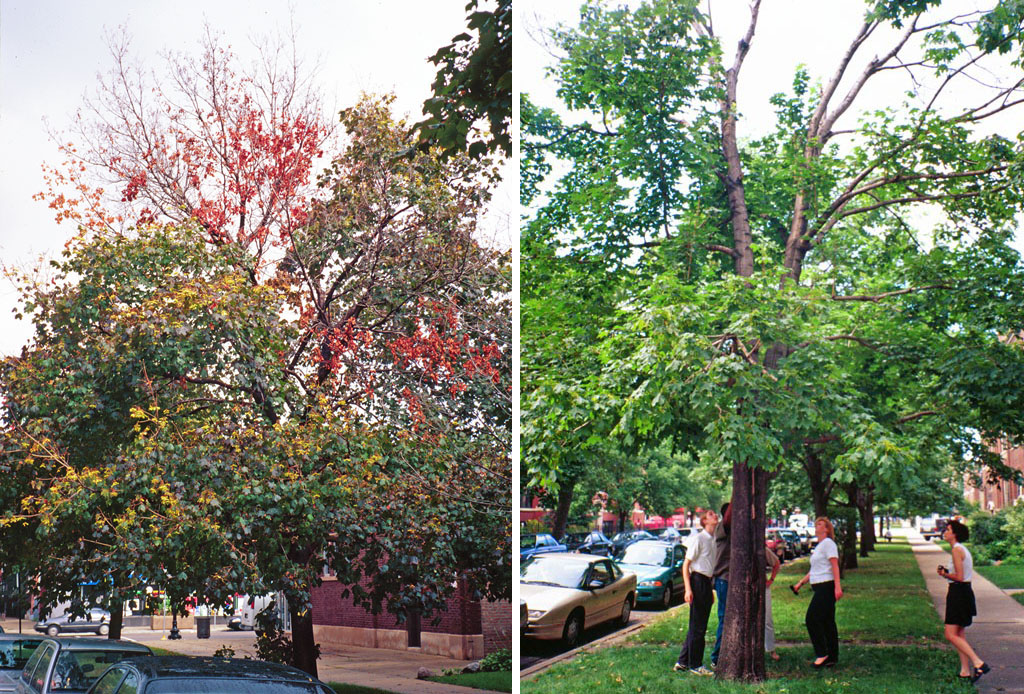

Fig. 7. Oviposition pits edged with chew marks (top and bottom). Image courtesy of Kenneth R. Law, USDA APHIS PPQ, Bugwood.org (top); Joe Boggs, Ohio State University, Bugwood.org (bottom).

Fig. 8. Frass on tree crotch resulting
from larvae feeding. Image courtesy of Robert A. Haack, USDA Forest Service, Bugwood.org.
Life History
Mated females chew pit-like depressions into the bark of healthy host trees and lay one to two eggs within each pit. These “oviposition pits” often have visible chew marks around the outer edges (Fig. 7) and are typically circular and 1/2 inch (15 mm) in diameter but can range from circular to a narrow slit about 3/64 inch (1 mm) deep. Eggs hatch within 2 weeks, and the emerging larvae bore into the tree to feed on living tissue (phloem and cambium) for several weeks before tunneling further into the woody tree tissue (xylem) to feed and develop over the winter. As they feed, the developing larvae create tunnels or galleries and push sawdust-like excrement (frass) from the tree (Fig. 8). The developing larvae undergo up to 13 molts (instars). When mature, each larva will form a pupal case where it will remain for 13 to 24 days and emerge from the tree as an adult by chewing a perfectly round exit hole, approximately 3/8 inch in diameter. Once emerged, adults typically remain on the same tree but can fly for 400 yards or more searching for a host tree or a mate. The adults live for about one month, feeding on leaves, twigs, and leaf stems (petioles) for 10 to 14 days before mating, laying eggs, and dying (APHIS, 2020). ALB overwinters in multiple life stages, and adults can be present from April to December, with summer to early fall being peak adult activity. Each mated female lays an average of 60 eggs in her lifetime. ALB’s life cycle (Fig. 9) lasts from 1 to 3 years, and in northern China, a single generation takes 2 years (CABI, 2021).
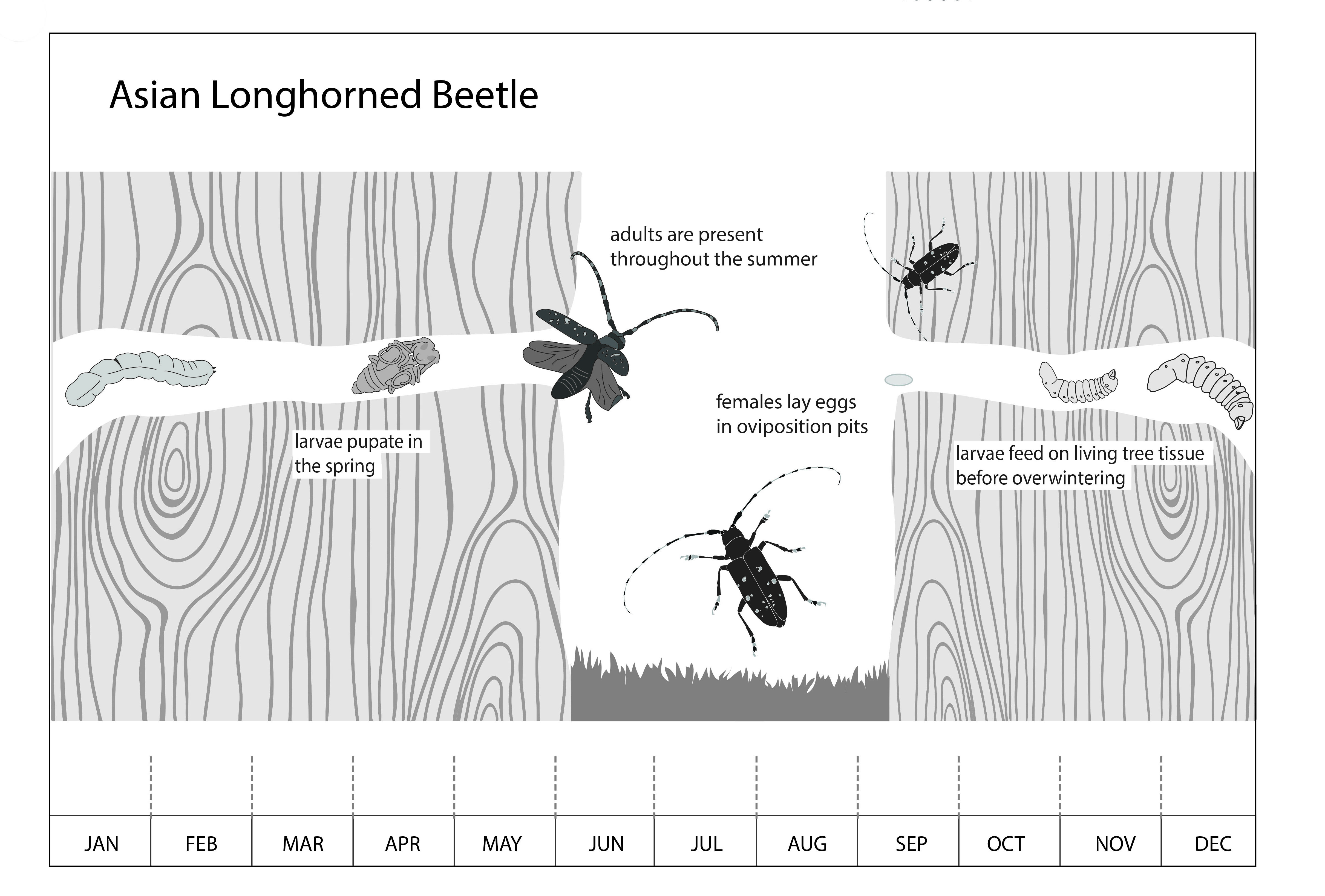

Fig. 10. ALB trap. Image courtesy of University of Arkansas Forest Entomology Lab , University of Arkansas, Bugwood.org.

Fig. 11. ALB damage. Image courtesy of Pennsylvania State University.
Monitoring and Prevention
Survey personnel use pheromone-baited traps, including interceptor traps (Fig 10), to monitor for ALB, and the U.S. Department of Agriculture has employed ALB scent-detection dogs in some areas where ALB occurs to help combat this pest. Citizen reports have been important in aiding eradication efforts in each state where ALB has occurred.
Visually monitor for signs of ALB any time of year but particularly in late June to August when this pest is most active. (In general, August is a good month to assess tree health in your landscape.) In Utah, monitoring should focus on the adult beetle, as many of the signs and symptoms of ALB are similar to those caused by other species (Cooperative Agricultural Pest Survey [CAPS], 2013; Spears et al., 2020), and ALB’s life cycle mainly takes place under bark. Inspecting the tree at crown level is considered more effective than ground inspections but is less practical for homeowners. Use binoculars to scan the upper parts of the host tree canopy where infestations usually begin, and look for concave oviposition pit wounds on the bark (Fig. 11). Note that pits on smooth bark are easier to detect than those on rougher bark. Newer pits up to several weeks old appear reddish in color and darken over time. Beginning in early spring, look for weeping sap and frass emanating or protruding from the pits, protruding from bark cracks, or appearing in branch junctions or at the tree base. Note that frass is rarely abundant and is most visible shortly after it has been extruded from the tree. Also look for bark cracks, missing bark, and bark that appears sunken or raised with a hollow area underneath, indicating separation from the sapwood. If fallen branches or loose bark exists, remove the bark and look for signs of feeding galleries or tunneling in the outer sapwood (Fig. 12). Frass may be present in the galleries. Beginning in the warmer months, look for circular 1/4 to 9/16 inch (6 to 14 mm) exit holes on host trees, about the diameter of a No. 2 pencil and at least 1 inch (2.5 cm) deep (Fig. 12). Most holes are visible for several years. When leaves are present, look for signs of adult feeding damage (Fig. 13) that includes chewed primary and secondary leaf veins, stripped outer tissue of twigs and petioles, and leaves that have fallen prematurely to the ground (APHIS, 2020).


Native Utah lookalikes (Fig. 14) include the whitespotted sawyer (Monochamus scutellatus), which has a white scutellum and dull bronze-black body, and the banded ash borer (Neoclytus caprea), whose antennae are shorter, and its wings only partially cover the body (CAPS, 2013).

Management
ALB has not been detected in Utah, so there is no current need for its control, and no approved control method for ALB exists at this time. The management options listed below are provided should this pest be detected in Utah in the future. The most practical approach is to prevent occurrences and, when detected, eradicate individuals before they spread to new areas. As of 2002, in an effort to help prevent new introductions of ALB and other invasive wood-boring pests, U.S. international shipping regulations require wooden packing materials to be chemically treated or kiln dried (CABI, 2021). If you suspect ALB in Utah, kindly contact the UPPDL or the UDAF
Cultural Methods
- Don’t move firewood. Larvae and adults can survive within firewood, and transporting firewood is a pathway for their spread into new areas. As a general rule, keep firewood inside county lines, and buy it where you burn it.
- Maintain tree health and vigor, as healthy trees may be more resistant to pests. Water and prune as needed, and fertilize when necessary. Take care to avoid injuring tree bases when mowing. Look for unseasonable yellowing leaves, or dropped or dying branches, which may indicate the tree is experiencing stress.
- Remove and chip or burn infested trees.
Biological Methods
ALB has no known natural predators in North America, and the natural enemies (parasitoids and predators) that keep ALB populations under control in its native range are unlikely to gain approval in the U.S. due to their generalist behaviors
Chemical Methods
No approved chemical control of ALB exists at this time. Imidacloprid has been shown to be effective against foraging adults and larvae (APHIS Plant Protection and Quarantine [PPQ], 2017), but the ability of systemic insecticides to reach lethal doses in tree crowns (where early infestations typically occur) has been questioned by some scientists and may negatively affect nontarget species, including pollinators (Meng et al., 2015)
References and Further Reading
-
Animal and Plant Health Inspection Service, Plant Protection and Quarantine (APHIS PPQ). (2017). Questions and answers: Asian longhorned beetle insecticide treatments. U.S. Department of Agriculture.
-
APHIS. (2020). Asian longhorned beetle (ALB). U.S. Department of Agriculture.
-
APHIS. (2021). Asian longhorned beetle. U.S. Department of Agriculture.
-
CABI. (2021). Anoplophora glabripennis (Asian longhorned beetle) [Fact sheet]. In Invasive Species Compendium. CAB International.
-
Cooperative Agricultural Pest Survey (CAPS). (2013). Anoplophora glabripennis (Motchlsky) [Fact sheet 3090]. Purdue University. Download.ceris.purdue.edu/file/3090
-
Department of Environmental Conservation. (n.d.). Asian longhorned beetle (ALB). New York State Department of Environmental Conservation.
-
Global Invasive Species Database. (2022). Species profile: Anoplophora glabripennis. Invasive Species Specialist Group.
-
Meng, P. S., Hoover, K., & Keena, M. A. (2015). Asian longhorned beetle (Coloptera: Cerambycidae), an introduced pest of maple and other hardwood trees in North America and Europe. Journal of Integrated Pest Management 6 (1). https://doi.org/10/1093/jipm/pmv003
-
Moorwood, W. D., Neiner, P. R., McNeil, J. R., Sellmer, J. C., & Hoover, K. (2003). Oviposition preference and larval performance of Anoplophora glabripennis (Coleoptera: Cerambycidae) in four eastern North American hardwood tree species. Environmental Entomology 32, 1028-1034.
-
Ohio Department of Agriculture. (n.d.). Asian longhorned beetle (ALB). Division of Plant Health. www.agri.ohio.gov/divisions/plant-health/invasive-pests/alb
-
Paine, T. & Hoddle, M. (n.d.). Asian long-horned beetle. Center for Invasive Species Research, University of California Riverside.
-
Spears, L. R., Alston, D. G., Brennan, E., Cannon, C., Caputo, J., Davis, R., Hebertson, L., Keyes, C., Malesky, D., McAvoy, D., Mull, A. M., Ramirez, R. A., Rodman, T. M., & Watson, K. (2020). First detector guide to invasive insects: Biology, identification & monitoring. Utah State University Extension
Related Research