Balsam Woolly Adelgid
[Adelges piceae (Ratzeburg)]
Revised April 2023
Liz Rideout1 • Kate Richardson1 • Diane Alston1 (No longer at USU) • Ryan Davis3 (No longer at USU) • Darren McAvoy2 Lori Spears5 (No longer at USU) • Danielle Malesky3 • Liz Herbertson3 • Colleen Keyes4
1USU Dept. of Biology; 2USU Dept. of Wildland Resources; 3USDA Forest Service; 4Utah Division of Forestry, Fire & State Lands, 5USDA APHIS
Quick Facts
- Balsam woolly adelgid (BWA) was first detected in the U.S. in 1908. It now infests true firs along both U.S. coasts and in several Interior West states.
- BWA was first observed killing subalpine fir trees in northern Utah forests in 2017 and is now confirmed in 10 counties.
- Due to its small size, its presence is difficult to detect until a tree is heavily infested and displays advanced symptoms (canopy decline, branch and node swelling).
- Controlling BWA with insecticides in forested landscapes is limited by high cost and other factors. The most viable management option is removing infested trees.
- BWA on individual or small groups of trees can be managed with insecticides applied during late spring/early summer and fall when the crawler (juvenile) stage is active.


The balsam woolly adelgid (BWA), Adelges piceae (Ratzeburg) (Hemiptera: Adelgidae), is a tiny sapsucking insect that was introduced to North America from Europe (Fig. 1). In the U.S., it is a pest of true firs along both U.S. coasts and in several Interior West states, where it impacts the health of firs in forested habitats, Christmas tree production, and potentially urban areas.
In some areas of the U.S., BWA has devastated fir populations in both forests and Christmas tree farms. In Utah, subalpine fir (Abies lasiocarpa) is a highly susceptible host tree. White fir (A. concolor) is also a host but is more tolerant. Douglas fir (Pseudotsuga menzisii) is not a true fir and, therefore, is not affected by BWA. The USDA Forest Service Forest Health Protection (FHP) team in Ogden first detected and confirmed BWA in 2017 in Farmington Canyon (Fig. 2) and near Powder Mountain Ski Resort. It is now confirmed in Box Elder, Cache, Rich, Weber, Morgan, Davis, Summit, Salt Lake, Wasatch, and Utah counties. Subalpine fir can typically be found at elevations of 5,900–11,400 feet (1,798–3,475 m) and often cohabitate with species such as white fir, Engelmann spruce (Picea engelmannii), and quaking aspen (Populus tremuloides).
Tree Injury and Symptoms
Subalpine fir is at high risk for BWA attack in Utah. Mature trees, 4 inches (10 cm) or more in diameter, seem most susceptible, but saplings may also be affected. In the West, stem (trunk) infestations are common at sites with higher-quality soils and lower elevations. Crown attacks occur more often at sites with poorer soils and higher elevations. Damage is most severe during the first decade of infestation in an area. It is unclear how long BWA may persist in an affected habitat, though pest impacts will remain indefinitely. BWA is most visible in the fall in Utah. Common tree symptoms include:
- Yellowing, then bronzing, of needles on the innerbranches that remain attached to the tree branch(called branch flagging) (Fig. 3).
- Loss of apical dominance (curling outward of crownbranches called top curl, or general upper crowndeath) (Fig. 3).
- Reduced growth, stunted trees/branches, particularlyin saplings (Fig. 4).
- Woolly material evident on tree bole near the treebase (Fig. 5).
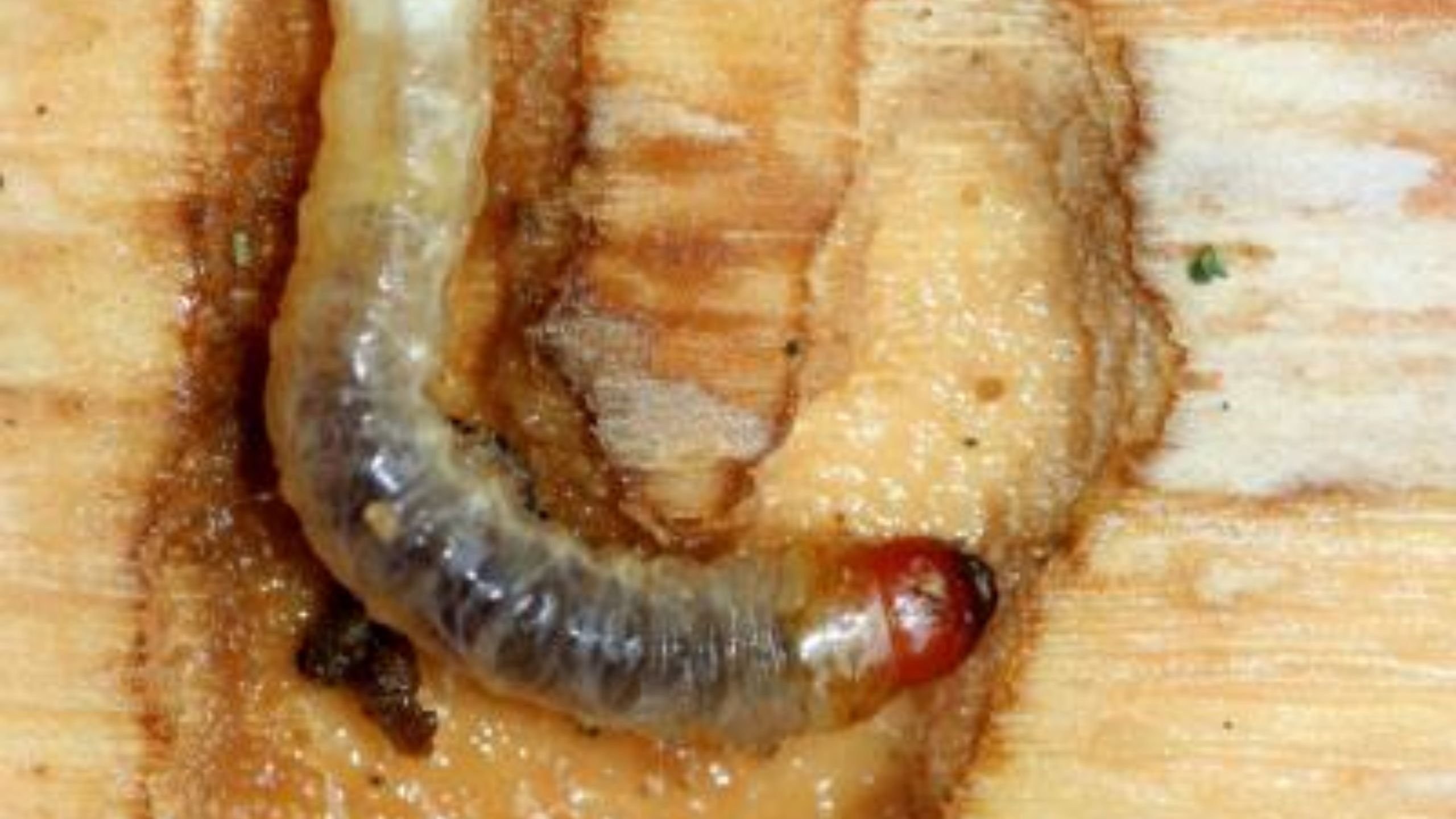
- Abnormal swelling of branch nodes and buds called“gouting” in response to adelgid feeding (Fig. 6).
- Reduced cone production and poor standregeneration.




Stem or bole infestations tend to be more serious than crown infestations. Infestation can also result in wide, irregular growth rings and reddish, brittle wood called “rotholz,” though this symptom has not been observed in the Intermountain West. Host responses to BWA feeding eventually cause decreased water flow to the crown, leading to drought-like symptoms and eventual tree death. Tree mortality typically occurs within 2-10 years of infestation, but heavy infestations can kill trees in 2-3 years.
Life History and Identification
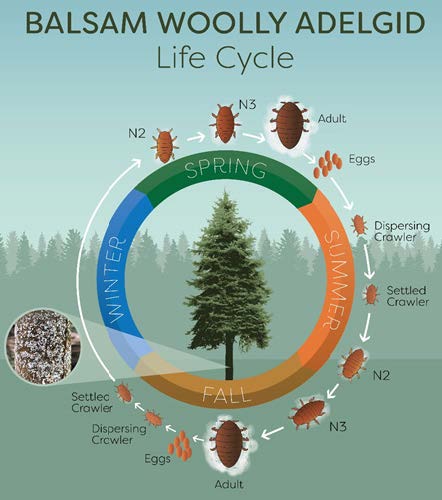
In its native range, BWA utilzes both spruce and fir species for different parts of its life cycle. However, in North America, BWA only utilizes true firs as its European spruce host is not present. BWA populations in North America are composed of only females reproducing without mating (parthenogenesis), because sexual reproduction requires the European spruce host. Depending on host species and climate, between two and four generations are typically observed in western North America, and two have been observed in northern Utah (Fig. 7).
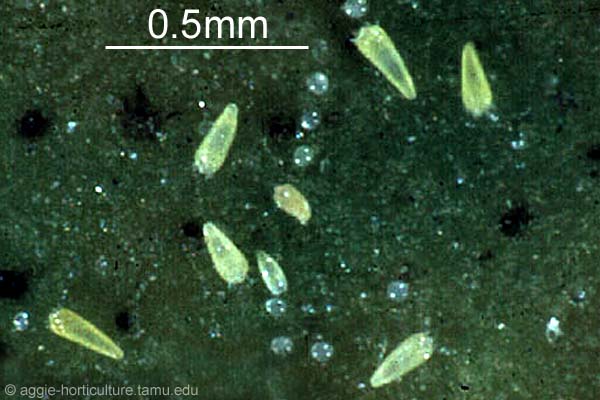
The egg incubation period is roughly 2 weeks and is followed by development into the first instar, the “crawler.” This is the only stage capable of motility and is roughly 0.4 mm in length (Fig. 8). Once hatched from the egg, crawlers wander the bark in search of a suitable feeding site. During this unsettled period, crawlers may be passively dispersed among trees and across forested landscapes primarily by wind and occasionally by animals. Upon choosing a feeding site, the crawler will insert its stylet and begin feeding.

Monitoring
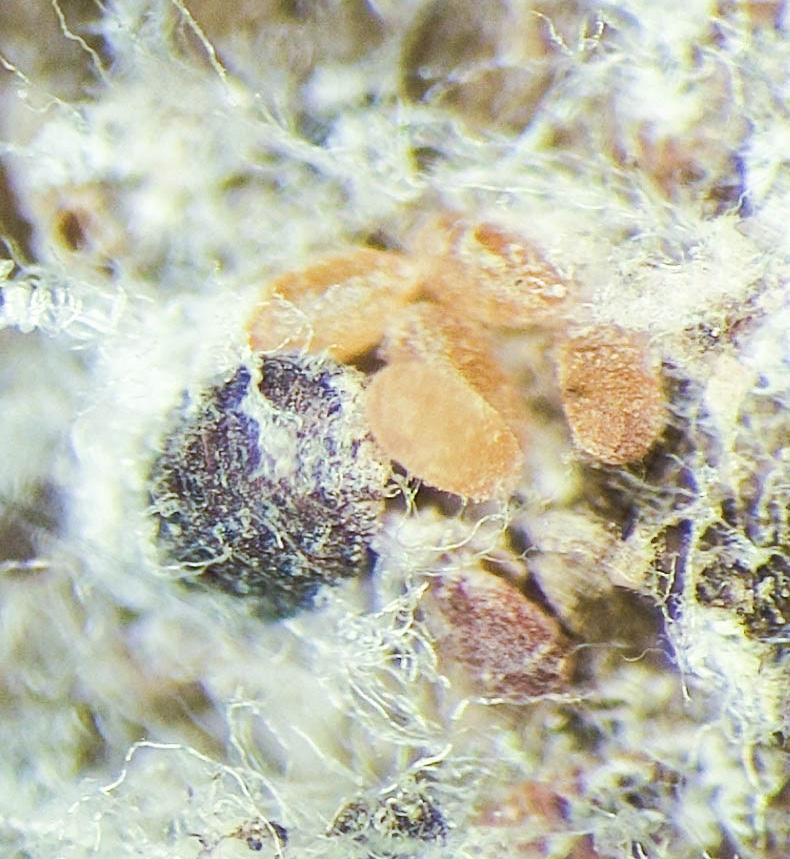
Rapid and reliable detection of BWA has proved challenging due to the insect’s small body size and immobile nature. Detection on a tree typically occurs after the population has grown dense enough to form visible masses of wool on the tree bole (Fig. 10) or injury symptoms such as branch gouting are present.
One detection method is regularly removing bark pieces followed by viewing under a dissecting microscope or high-powered hand lens (Fig. 11). This approach can be effective but is time consuming, requires specific equipment, and may be harmful to the tree’s bark over time.
Targeted monitoring of dispersing BWA crawlers using sticky cards has shown potential for being a simple and accessible monitoring tool for tracking the timing of crawler dispersal and potentially population densities. Dispersing crawlers may be detected by placing sticky cards horizontally (sticky side facing up) on a stake or post below the canopy of the infested tree. To reduce bycatch, attach a fine mesh screen to the card before deploying on the stake (Fig. 12). For the greatest reduction in bycatch, the mesh screen should have openings between 1 mm and 1 cm. Cards should be regularly removed, replaced, and inspected for crawlers using a hand lens. Placing these cards near fir trees between April-June and August-September can provide location-specific valuable insight into timing management actions with periods of crawler dispersal.


Management
Cultural Control
Completely removing BWA from western ecosystems is unrealistic as they are widespread and disperse by wind. At the forest scale, the most effective tactics to reduce BWA damage include:
- Selectively removing heavily infested trees in winterwhen crawlers are inactive.
- Considering prevailing winds when establishingcutting boundaries.
- Growing fir on short rotation cycles.
- Favoring nonhost tree species and geneticallyresistant strains or hybrids through selective harvestingand planting.
- Using stand management techniques to promotestand vigor.
- Limiting the movement of infested firewood.
Several hazard/risk rating systems have been developed for forest managers to optimize scouting and management programs for BWA (Ragenovich & Mitchell 2006; Hrinkevich et al., 2016). Fir species, site elevation, and soil/stand conditions are the primary factors driving stand susceptibility. Adding to the challenge of managing this insect is the lack of market value for subalpine fir; recovering treatment costs through selling sawlogs is, therefore, infeasible.
Biological Control
Thirty-three predator species have been released and monitored for establishment and effectiveness at BWA management between 1933 and 1969. Of these, six species successfully established in both the U.S. and Canada: three fly species (families: Chamaemyiidae and Cecidomyiidae) and three beetle species (families: Coccinelidae and Derodontidae). Despite establishment and evidence of predation on BWA, there have been no confirmed reductions in BWA populations or decreases in tree mortality associated with any of these biological control agents.
Chemical Control
Due to their small size, protected feeding sites, and waxy coatings, aerial insecticide applications do not provide coverage adequate for large-scale insect control. A thorough insecticide application to high-value trees using a high-pressure system can provide BWA control in areas such as ski resorts, cabin properties, campgrounds, tree farms, and urban settings. Chemical treatments can prove useful when combined with other management techniques, such as nonhost tree planting, but alone would be indefinite, costly, and ongoing.
Table 1. Insecticides for Balsam Woolly Adelgid Control
| Commercial | |
|---|---|
| IRCA Mode of Action Groups | Insecticide |
| Carbamate (group 1A) | Carbaryl |
| Neonicotinoid (group 4A) | Dinotefuran Thiamethoxam |
| Organophosphate (group 1B) | Chlorpyrifos* |
| Pyrethroid (group 3A) | Bifenthrin Esfenvalerate |
| Tetronic & tetramic acid derivatives (group 23) | Spirotetramat |
| Homeowner | |
| IRCA Mode of Action Groups | Insecticide |
| Neonicotinoid (group 4A) | Imidacloprid |
| Pyrethroid (group 3A) | Permethrin |
| Potassium salts of fatty acids; petroleum distillate | Insecticidal soap/oil |
| Sucrose esters | Sucrose octanoate |
*Requires a Utah pesticide applicator license to purchase and apply.
For Utah, the current recommendation is to target crawlers with a residual insecticide, horticultural oil, or soap in summer and/or fall. Specific timing of application to infested trees will depend on temperature, elevation, and location. To appropriately time insecticide applications, monitor for the crawler stage in late spring/early summer and/or fall. Timing applications to coincide with peak crawler activity is more important when using soaps or oils compared to longer-residual chemicals like the pyrethroids. Be aware of plant injury associated with soap or oil application before deciding to apply one of these tools. Table 1 displays available insecticide control options.
Using systemic neonicotinoids may provide control of BWA; however, this has not been confirmed by research. Systemic neonicotinoids are transported through the sapwood via water uptake. Moderate to severe damage from BWA will reduce water flow within the tree, limiting efficacy. If found effective, systemic neonicotinoids would be best used for new to moderate infestations, or preventively if infested trees are nearby and spread is anticipated.
Implications to Forest Health
Widespread mortality of subalpine fir is already occurring at some locations in northern Utah. In many cases, there are few other tree species to occupy the growing site. This problem increases the potential for BWA to inflict great ecological damage through increased erosion, decline in watershed health, loss of wildlife and their habitat, and reduction in recreational value. Additionally, potential dying and dead fir adding to fuel loading in forest landscapes is a high concern. True fir species are known for their capacity to retain dead green and dry needles in canopies over long periods, likely influencing fire severity and behavior.
A Utah partnership has been formed to implement survey, research, education, and management efforts for BWA. Members of concerned stakeholders include the United States Department of Agriculture (USDA) Forest Service; the Utah Division of Forestry, Fir, and State Lands; Utah State University (USU) Extension; USDA Animal and Plant Health Inspection Service; and ski resorts (Fig. 13). This group is coordinating efforts to secure grant funding to study BWA and its impact in Utah and to develop public educational resources.

balsam woolly adelgid damaged sites in Farmington Canyon, September, 2017.
References
-
Davis, G. A., Lowrey, L., Eckberg, T., Hicke, J. A., & Smirnova, E. (2022). Characterizing balsam woolly adelgid infestations and associated tree mortality in Idaho. Journal of Forestry, 120(4), 361–378. https://doi.org/10.1093/jofore/fvac007
-
Hastings, F., Hain, F., Mangini, A., & Huxster, W. (1986). Control of the balsam woolly adelgid (Homoptera: Adelgidae) in fraser fir Christmas tree plantations. Journal of Economic Entomology, 79, 1676–1680. https://doi.org/10.1093/jee/79.6.1676
-
Hrinkevich, K. H., Progar, R. A., & Shaw, D. C. (2016a). A severity rating system for evaluating stand-level balsam woolly adelgid (Hemiptera: Adelgidae) damage in two abies species in western North America. Forest Science, 62(2), 181–189. https://doi.org/10.5849/forsci.15-025
-
Hrinkevich, K. H., Progar, R. A., & Shaw, D. C. (2016b). Climate risk modelling of balsam woolly adelgid damage severity in subalpine fir stands of western North America. PLoS ONE, 11(10), e0165094. https://doi.org/10.1371/journal.pone.0165094
-
Lass, L. W., Cook, S. P., Shafii, B., & Prather, T. S. (2014). Development of a dispersal model for balsam woolly adelgid, Adelges piceae Ratzeburg (Hemiptera: Adelgidae), to facilitate landscape-level management planning. International Journal of Forestry Research, e519010. https://doi.org/10.1155/2014/519010
-
Montgomery, M. E. (2014). Balsam woolly adelgid. USDA Forest Service. https://www.fs.usda.gov/nrs/pubs/jrnl/2014/nrs_2014_montgomery_001.pdf
-
Ragenovich, I., & Mitchel, R. (2006). Balsam woolly adelgid [Forest insect & disease leaflet 118]. USDA Forest Service. https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/fsbdev2_043667.pdf
Related Research